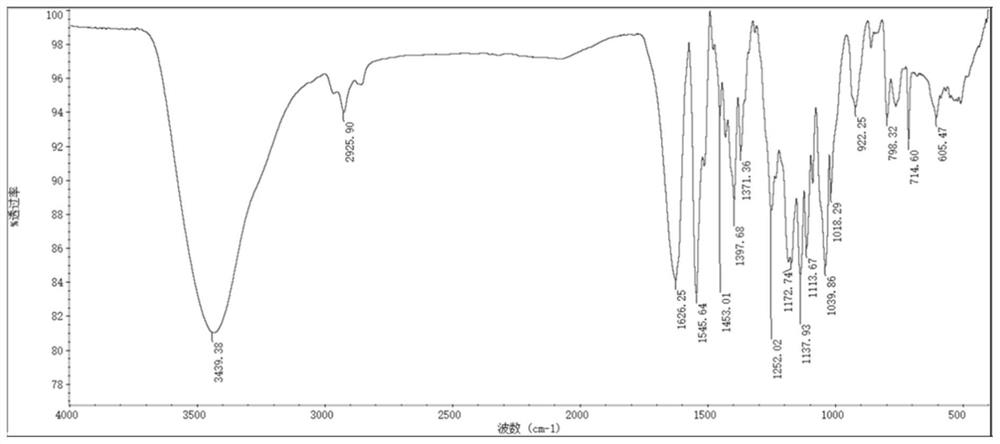
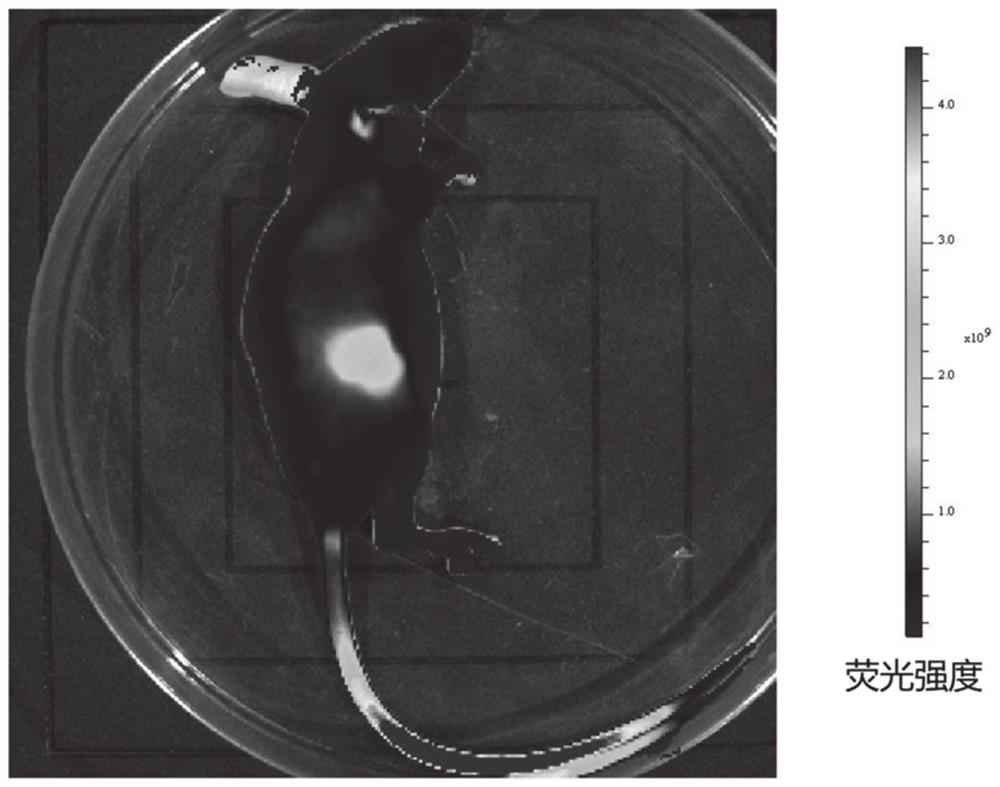
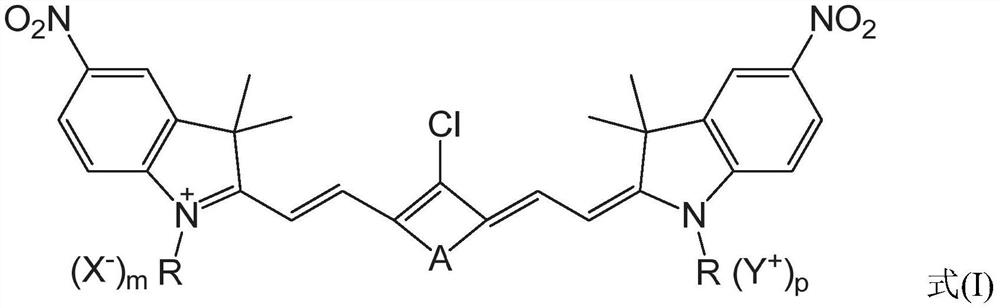
A heptamethine nitroindole cyanine dye and the preparation method and application of the dye
A technology for nitroindocyanine and nitroindole derivatives, which is applied in the directions of methine/polymethine dyes, organic dyes, chemical instruments and methods, etc., can solve the problem of high price, high consumption of organic solvents, high purity lower problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] Example 1 Synthesis of 2,3,3-trimethyl-4-nitroindole
[0156] Dissolve p-nitrophenylhydrazine, 3-methyl-2-butanone, and anhydrous sodium acetate at a molar ratio of 1:1.1:1.5 in acetic acid, and react under reflux and stirring for 8 hours. The reaction solvent was removed by rotary evaporation, and then a mixed solution of water and methanol with a volume ratio of 9:1 was added to dissolve the remaining substances. The resultant was filtered, and then exposed to crystallization at room temperature for 48 hours to obtain crystal 2,3,3-trimethyl-4-nitroindole.
Embodiment 2
[0157] Embodiment 2 synthetic compound 53
[0158] Compound 53 was synthesized according to the following route:
[0159]
[0160] 1) Synthesis of 2,3,3-trimethyl-1-(butane)-nitroindole
[0161] The 2,3,3-trimethyl-nitroindole and 4-bromobutane obtained in Example 1 were added into the reactor at a molar ratio of 1:1.5, and the reactor was sealed and then evacuated to 10 Pa. The reaction system was heated to 110° C. and stirred for 8 hours, then cooled to room temperature. The obtained product was filtered with suction and used directly for the next reaction.
[0162] 2) Synthesis and purification of compound 53
[0163] 2-Chloro-1-formyl-2-chloro-1-formyl- 3-Hydroxymethylenecyclopentene. After completely dissolving with methanol, the reaction system was heated to 75° C. for 24 hours under closed conditions, then cooled to room temperature, and placed in a 4° C. refrigerator for 24 hours. Petroleum ether was added, then allowed to stand and filtered with suction. The...
Embodiment 3
[0165] Embodiment 3 synthetic compound 26
[0166] Compound 26 was synthesized according to the following route:
[0167]
[0168] 1) Synthesis of 2,3,3-trimethyl-1-(p-methylbenzoic acid)-nitroindole
[0169] In the reactor, add the 2,3,3-trimethyl-nitroindole and p-bromomethylbenzoic acid obtained in Example 1 with a molar ratio of 1:1.5, and vacuumize the reactor to 20Pa after sealing . The reaction system was heated to 110° C. and stirred for 12 hours, then cooled to room temperature. The obtained product was filtered with suction and used directly for the next reaction.
[0170] 2) Synthesis and purification of compound 26
[0171] Add 2,3,3-trimethyl-1-(p-methylbenzoic acid)-nitroindole molar ratio of 1:2.5 2-chloro-1- Formyl-3-hydroxymethylenecyclohexene. After completely dissolving with methanol, the reaction system was heated to 75° C. for 24 hours under closed conditions, then cooled to room temperature, and placed in a 4° C. refrigerator for 24 hours. Petro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com