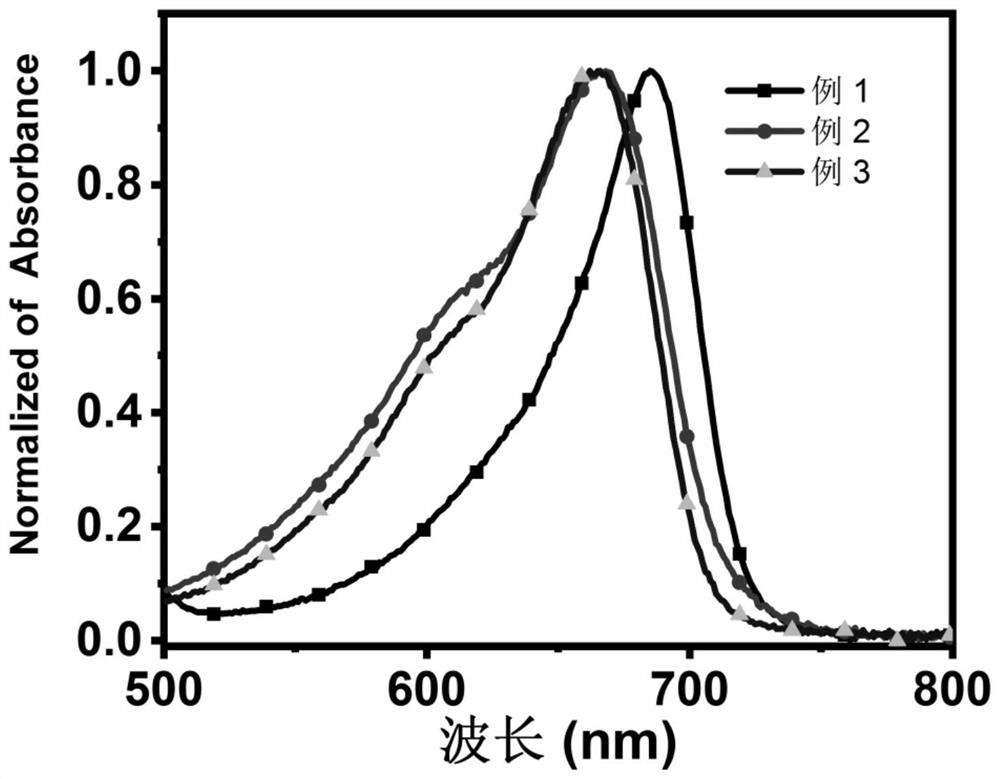
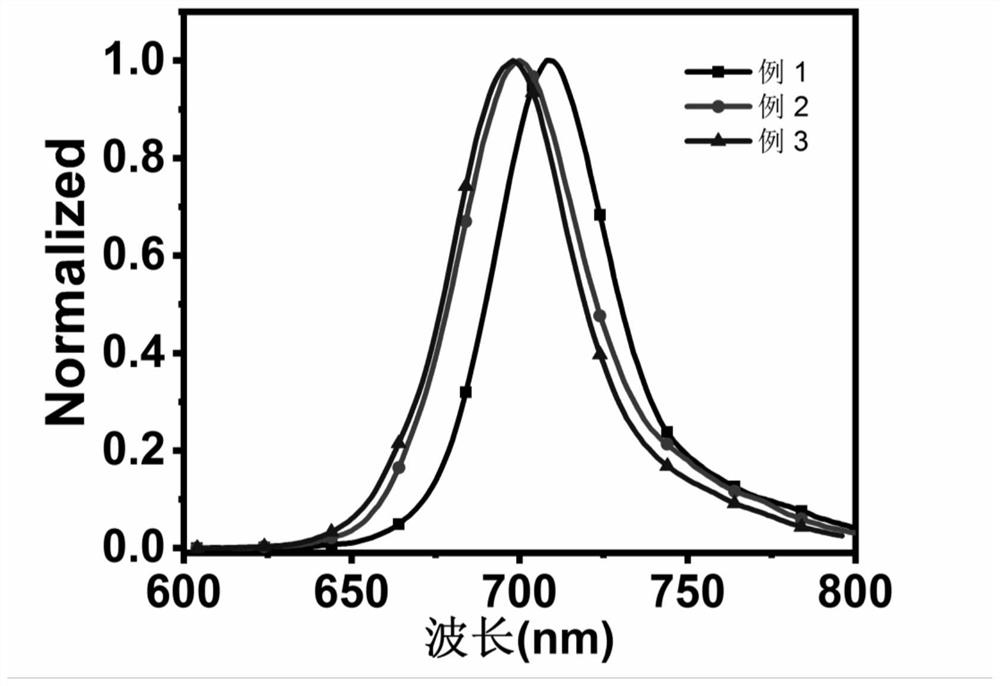
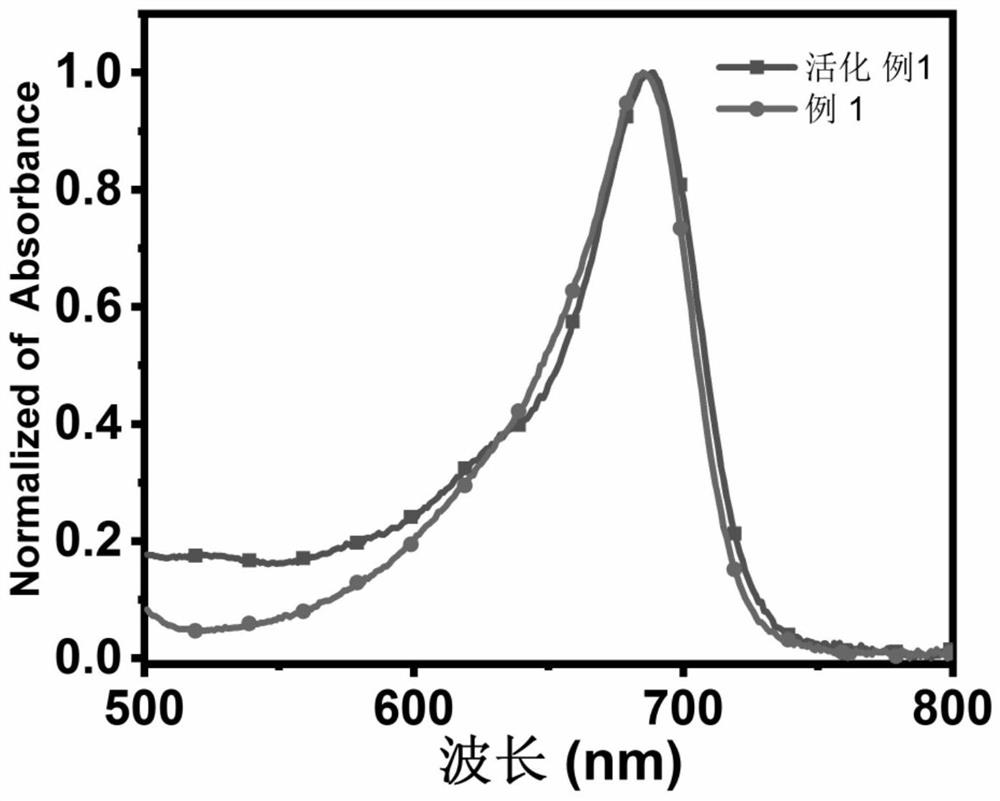
Amide-substituted azaindole-pentamethine cyanine dye as well as synthesis method and application thereof
An azaindole and cyanine dye technology, which is applied in the field of biological identification reagents, can solve the problems of difficulty in entering biological tissues, reduced detection efficiency, limited excitation time, etc., and achieves flexible labeling methods, strong applicability, and good water solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1 manufactures compound 1
[0056]
[0057] The structural formula of compound 1
Embodiment 11
[0059]
[0060] To a solution of p-hydrazinobenzenesulfonic acid (5.00 g, 26.6 mmol, 1.0 eq) in acetic acid at room temperature, was added 3-methyl-2-butanone (5.70 mL, 53.1 mmol, 2.0 eq), refluxed After 4h, stop the reaction, filter, wash with ethyl acetate three times, dissolve the solid in 30mL of methanol, slowly add dropwise to a solution of potassium hydroxide (1.50g, 26.6mmol, 1.0eq) dissolved in 30mL of propanol, at room temperature Stir for 24h. After filtration, the target compound 1.1 (6.04 g, 21.8 mmol, Y=82%) was obtained.
Embodiment 12
[0062]
[0063] The compound of Example 1.1 (1.00g, 3.6mmol, 1.0eq) and 6-bromo-n-hexanoic acid (1.40g, 7.2mmol, 2.0eq) were dissolved in 10mL o-dichlorobenzene, refluxed for 24h under nitrogen, and the reaction was stopped . Add isopropanol and filter to obtain target compound 1.2 (1.14 g, 2.4 mmol, Y=67%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com