Heptamethane sulfonic indocyanine dye, preparation method and application thereof
A technology for heptamethinesulfonic acid-based indocyanines and dyes, which is applied in the directions of methine/polymethine-based dyes, organic dyes, chemical instruments and methods, and can solve the problems of high price, high consumption of organic solvents, low purity, etc question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
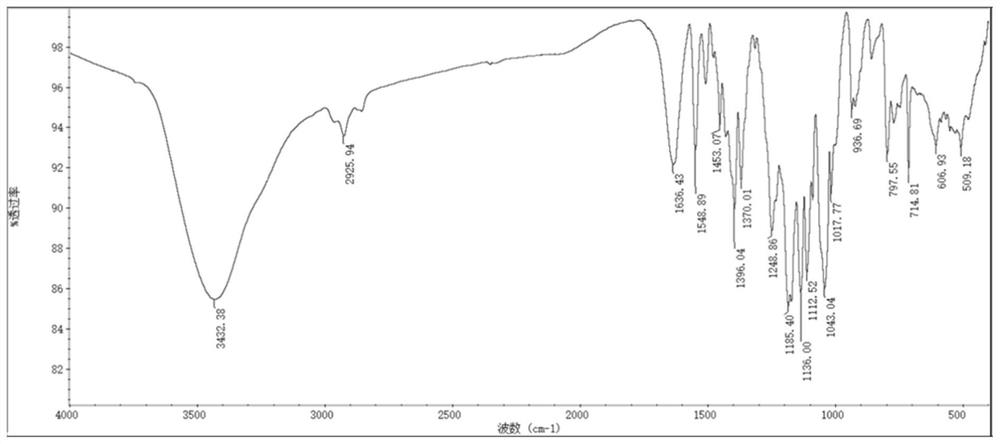
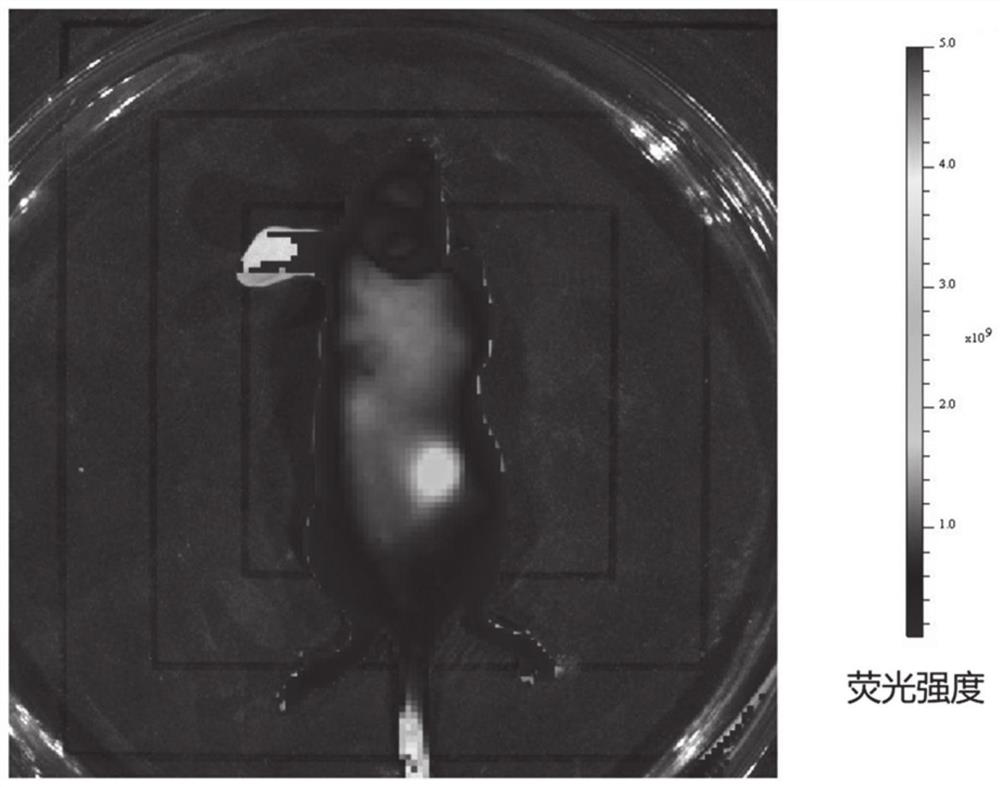
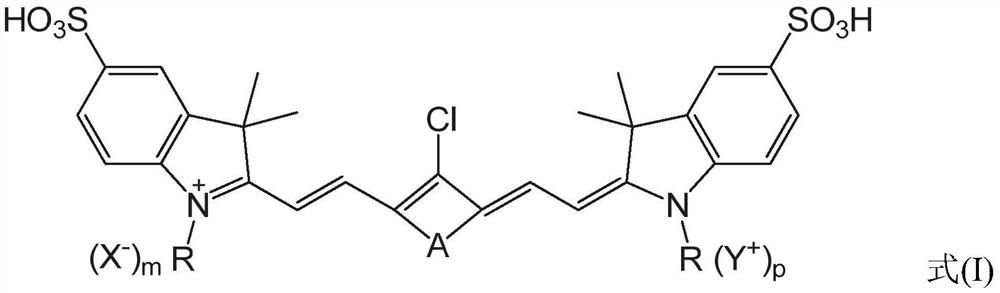
[0143] As mentioned above, the present application relates to a preparation method of heptamethinesulfonate indole cyanine dye, comprising the following steps: 2,3,3-trimethyl-sulfonate indole derivative and nucleophilic substitution The compound is reacted to obtain an organic ammonium salt; the organic ammonium salt and cycloalkene derivatives are mixed in an environment-friendly organic solvent for reaction, and after the reaction, an organic precipitant is added to the product to cool and stand overnight to obtain the heptamethinesulfonyl indole Cyanine dyes. The method has the advantages of short synthesis route, simple process, no catalyst, high yield, simple purification method, high atom utilization rate and less consumption of organic solvents, can greatly improve the preparation efficiency of such dyes, and realize low-cost mass production , It is of great significance in the production and application research of heptamethinesulfonate indole cyanine dyes.
[0144] ...
Embodiment 1
[0151] Example 1 Synthesis of 2,3,3-trimethyl-4-sulfonic indole
[0152] Dissolve p-sulfophenylhydrazine, 3-methyl-2-butanone, and anhydrous sodium acetate at a molar ratio of 1:1.1:1.5 in acetic acid, and react under reflux and stirring for 8 hours. The reaction solvent was removed by rotary evaporation, and then a mixed solution of water and methanol with a volume ratio of 9:1 was added to dissolve the remaining substances. The resultant was filtered, and then exposed to crystallization at room temperature for 48 hours to obtain crystal 2,3,3-trimethyl-4-sulfonylindole.
Embodiment 2
[0153] Embodiment 2 synthetic compound 53
[0154] Compound 53 was synthesized according to the following route:
[0155]
[0156] 1) Synthesis of 2,3,3-trimethyl-1-(butane)-sulfonyl indole
[0157] In the reactor, add 2,3,3-trimethyl-sulfonyl indole and 4-bromobutane obtained in Example 1 with a molar ratio of 1:1.5, and vacuumize the reactor to 10Pa after sealing . The reaction system was heated to 110° C. and stirred for 8 hours, then cooled to room temperature. The obtained product was filtered with suction and used directly for the next reaction.
[0158] 2) Synthesis and purification of compound 53
[0159] Add 2,3,3-trimethyl-1-(butane)-sulfonylindole molar ratio of 1:2.5 2-chloro-1-formyl- 3-Hydroxymethylenecyclopentene. After completely dissolving with methanol, the reaction system was heated to 75° C. for 24 hours under closed conditions, then cooled to room temperature, and placed in a 4° C. refrigerator for 24 hours. Petroleum ether was added, then allowe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com