A kind of gamma-glutamine synthetase mutant and its application
A technology of glutamyl methylamine and synthase, applied in the field of enzyme catalysis, can solve the problems of low enzyme specific activity, product-inhibitory industrialization constraints, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 The activity comparison of wild-type γ-glutamine synthetase
[0051] 1.1 Construction of γ-glutamine synthetase genes from two microbial sources
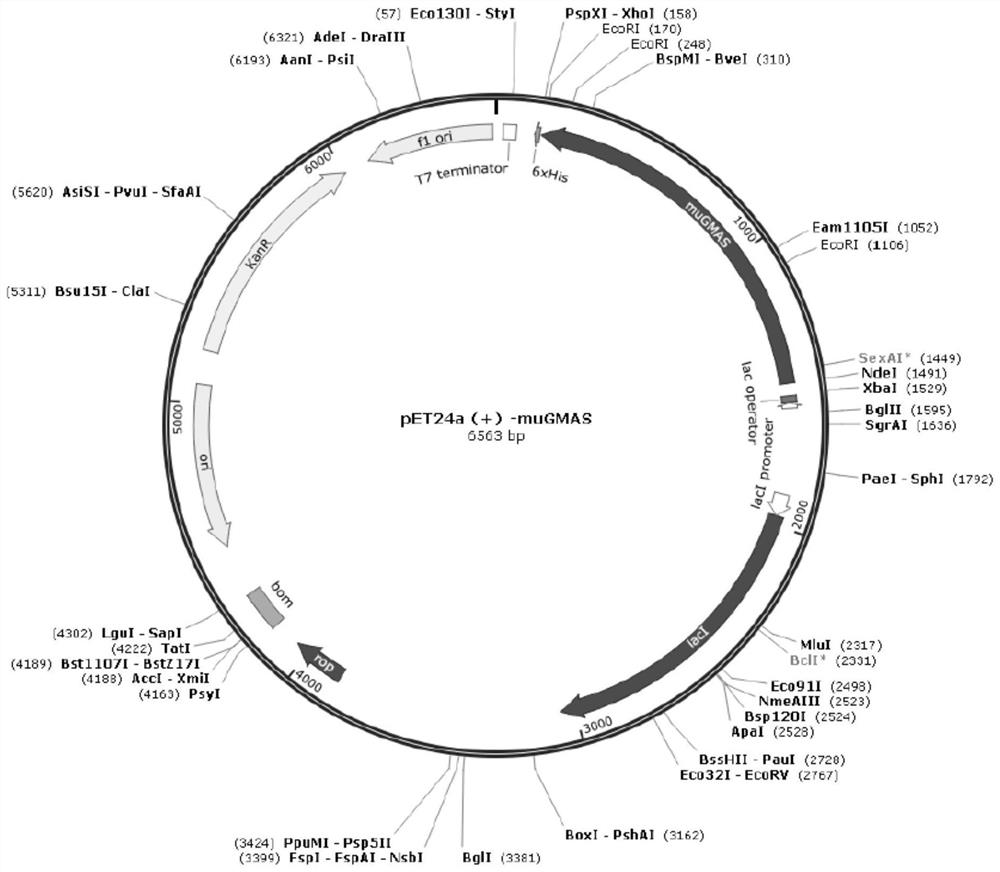
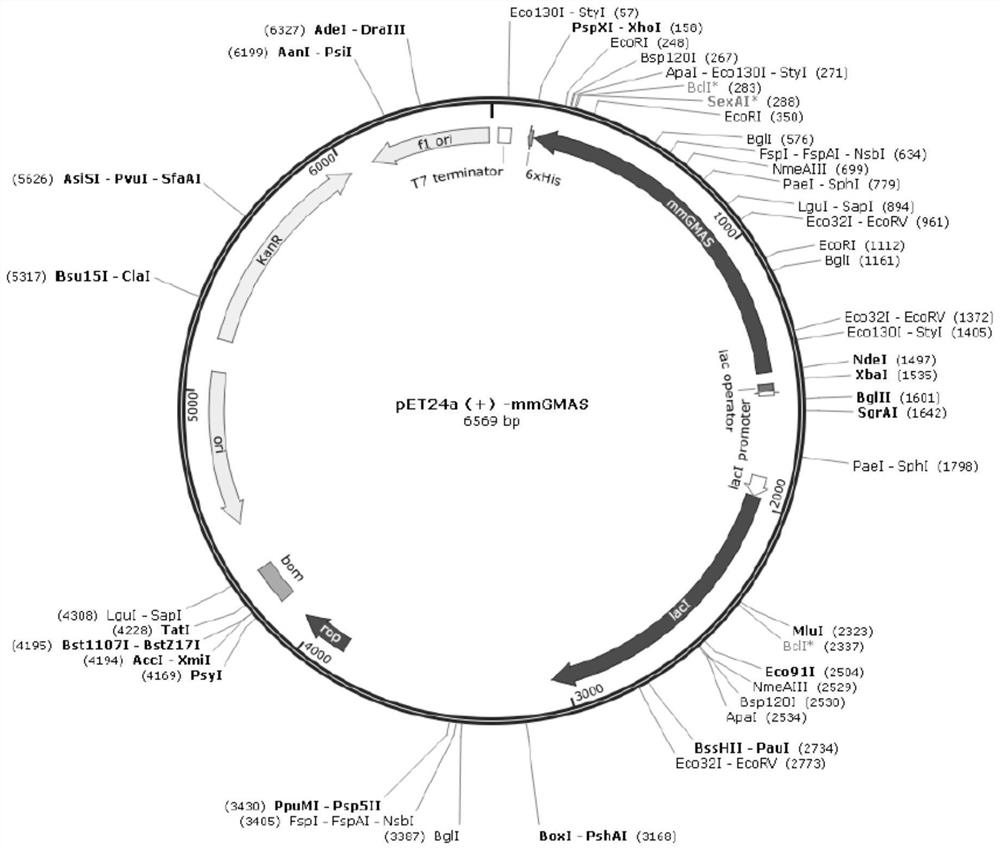
[0052] Whole-gene synthesis of γ-glutamine synthetase gene SEQ ID NO:2 from Methyloversatilis universalis (GenBank No. WP_008064112) and γ-glutamine synthesis from Methylovorus mays NO.9 (GenBank No. WP_008064112) Enzyme gene SEQ ID NO: 4, and restriction endonuclease sites NdeI and XhoI were designed at both ends of the gene respectively, subcloned into vector pET24a (+), and two wild-type gene recombinant plasmids pET24a-muGMAS were obtained (see figure 1 ) and pET24a-mmGMAS (see figure 2 ) were transformed into Escherichia coli expression host BL21(DE3) respectively to obtain recombinant Escherichia coli pET24a-muGMAS / BL21(DE3) and pET24a-mmGMAS / BL21(DE3).
[0053] 1.2 Shake flask fermentation of two microbial sources of γ-glutamine synthetase strains
[0054] Pick a single colony from the pET24a-muGMAS / BL...
Embodiment 2
[0062] Example 2 Construction of site-directed mutants and identification of their activity
[0063] 2.1 Construction of site-directed mutants
[0064] Using the gene sequence of SEQ ID NO: 3 as a template, the primer pairs muGMAS-152F / muGMAS-197R and muGMAS-210F / muGMAS-261R were used for PCR respectively, and two PCR products P1 and P2 were obtained by amplification respectively. The PCR primer sequences are as follows:
[0065] muGMAS-152F:
[0066] 5'-GTGACACCCTGTCTAAACCGAGCTACGACTACAAAGGTCTGTCTC-3';
[0067] muGMAS-197R:
[0068] 5'-CAGCACCCATTTTGGCGAAGGTGTAGTGGTCGCAAGAGGTCAGAGCGTCGGTCAAGGTGTAGTTGATTTCGAAC-3'.
[0069] muGMAS-210F:
[0070] 5'-GTTCGAAATCAACTACACCTTGACCGACGCTCTGACCTCTTGCGACCACTACACCTTCGCCAAAATGGGTGCTG-3';
[0071] muGMAS-261R:
[0072] 5'-CCAGTGGTAAGCCAGTTTAGACAGAGCCAGACCGGTTTTGTCAGATTTGTCTT CGAAC-3'.
[0073] 50μl PCR reaction system includes: 10ng pET24a-muGMAS plasmid template, 10pmol primer pair, 1 × KOD plus buffer, 0.2mM dNTP, 1.5mM MgSO 4 , 5...
Embodiment 3
[0087] Example 3 Synthesis reaction of L-theanine coupled with polyphosphoric acid ATP regeneration system
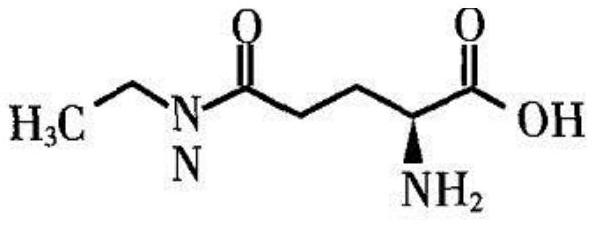
[0088] The reaction system is 1L, 200mM sodium glutamate, 450mM ethylamine hydrochloride, 30mM MgCl 2 , 5mM MnCl 2 , 5mM ATP, 75mM hexametaphosphoric acid, 3.5% of the polyphosphokinase production strain PET24a-cgPPK2-Mut41 / BL21 (DE3) fermentation freeze-thawed bacteria body reported in the patent document CN202010213070.0, 6.5% of the strain obtained in Example 2 Freeze-thaw pET24a-muGMAS-no21 / BL21 (DE3) cells, react at 37°C, 200rpm for 12h; add 200mM sodium glutamate, 50mM hexametaphosphate, 2.5% pET24a-muGMAS-no21 / BL21 ( DE3) Freezing and thawing the bacteria, controlling the pH to 7.0 during the reaction process, continuing the reaction for 20 hours, sampling and testing, 63.01g / L L-theanine was produced, the yield was over 90%, and the ee value of the product was greater than 99%.
[0089] In summary, the enzyme activity of the γ-glutamine synthetase mutant SEQ I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com