Desulfurizing agent for removing dibenzothiophene and 4, 6-dimethyl dibenzothiophene in oil product as well as preparation method and application of desulfurizing agent
A technology of dimethyl dibenzothiophene and dibenzothiophene, which is applied in the field of oil desulfurization in the petrochemical field, can solve the problems of cost increase, hydrogen consumption increase, octane number reduction, etc., and achieve the effect of good application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
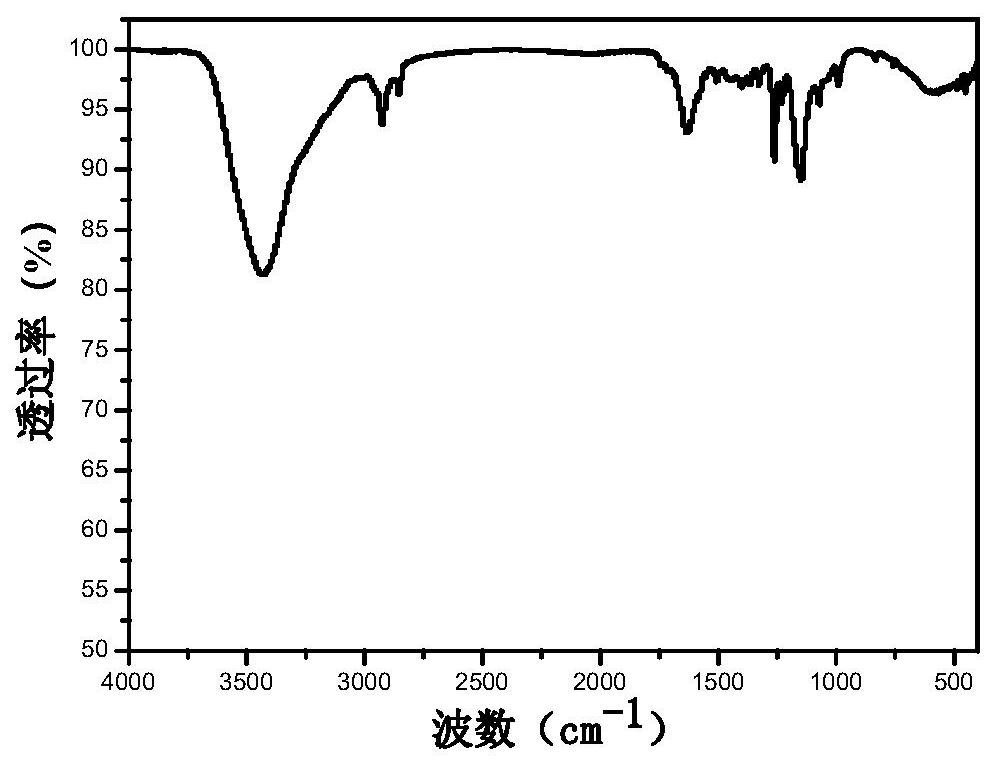
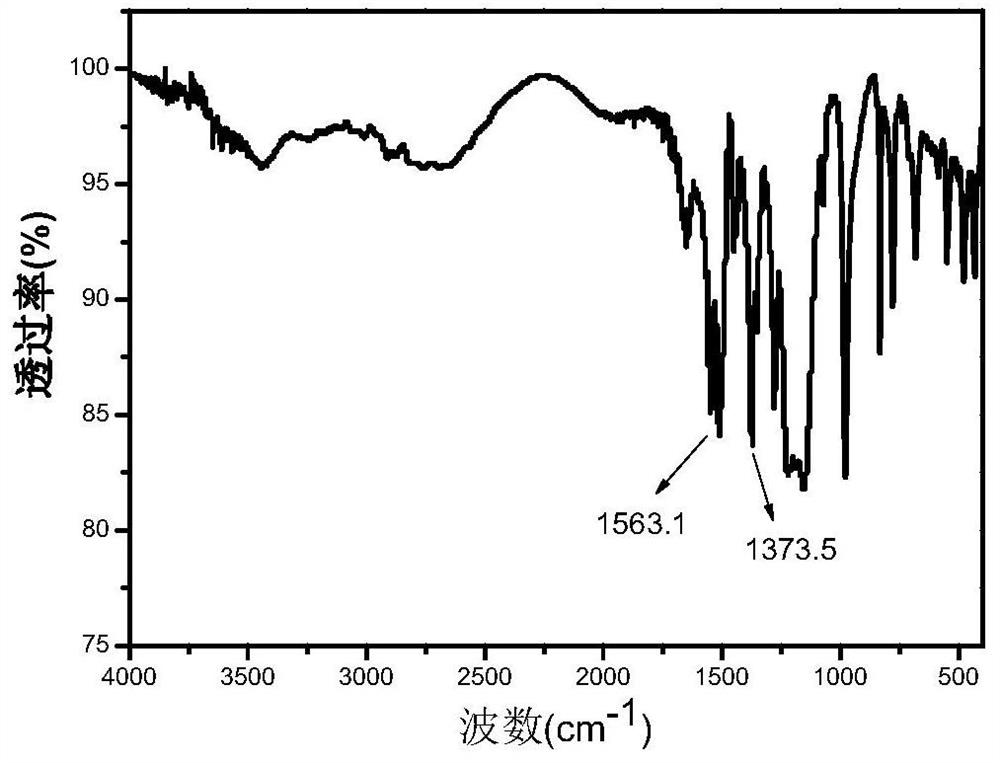
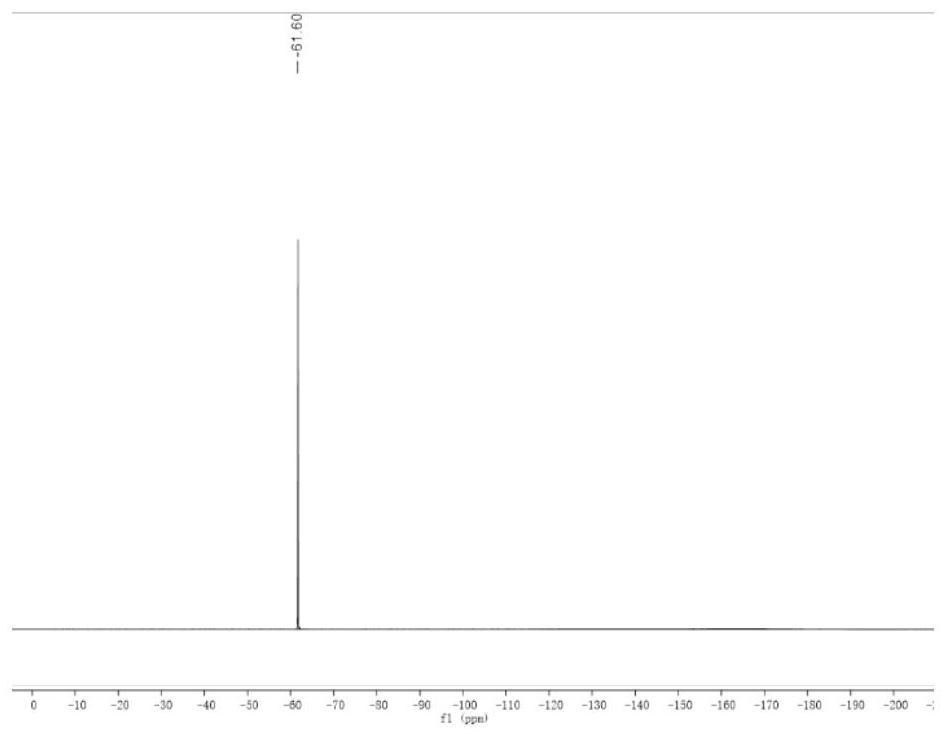
[0030] Using 3,5-bis(trifluoromethyl)pyrazole as raw material, dissolve 1.02g of 3,5-bis(trifluoromethyl)pyrazole in 4.5ml of concentrated sulfuric acid under ice-bath conditions, then drop slowly Add 2ml fuming HNO 3 , mix well to dissolve. Then react at 122°C for 15 hours; then cool naturally, pour the reaction solution into 300mL of ice water and let it stand for 4 hours, a white solid appears, then collect it by filtration, and keep it at room temperature for 48 hours to dry. Obtain pyrazole organic monomer (CF 3 ) 2 NO 2 PzH, 19 See the FNMR diagram for details image 3 , whose infrared spectrum is detailed in figure 1 .
[0031] Dissolve the pyrazole organic monomer 3,5-bis(trifluoromethyl)pyrazole obtained above in toluene (according to 1g of pyrazole organic monomer corresponds to 40ml of toluene), and add 0.84g of 1g monomer Ag 2 O, followed by reflux at 100°C for 5 hours. Filtrate while it is hot, cool naturally, a large amount of white crystals are precipi...
experiment example 1
[0035] Weigh 110 mg of desulfurizer, pour it into 30 ml of DBT and DMDBT solutions with a sulfur content of 100 mg / L, stir at room temperature for 50 hours, then centrifuge it, and test the residual thiophene concentration in the supernatant by ultraviolet absorption spectroscopy. The test results are as follows: Table 1 shows.
experiment example 2
[0037] Weigh 11 mg of desulfurizer, pour it into 30 ml of DBT and DMDBT solutions with a sulfur content of 10 mg / L, stir at room temperature for 50 hours, then centrifuge it, and test the residual thiophene concentration in the supernatant by ultraviolet absorption spectroscopy. The test results are as follows: Table 1 shows.
[0038] Table 1
[0039] The initial concentration Equilibrium concentration Desulfurization rate The initial concentration Equilibrium concentration Desulfurization rate DBT 100mg / L 2.5mg / L 97.5% 10mg / L 1.3mg / L 87% DMDBT 100mg / L 1.2mg / L 98.8% 10mg / L 1.1mg / L 89%
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap