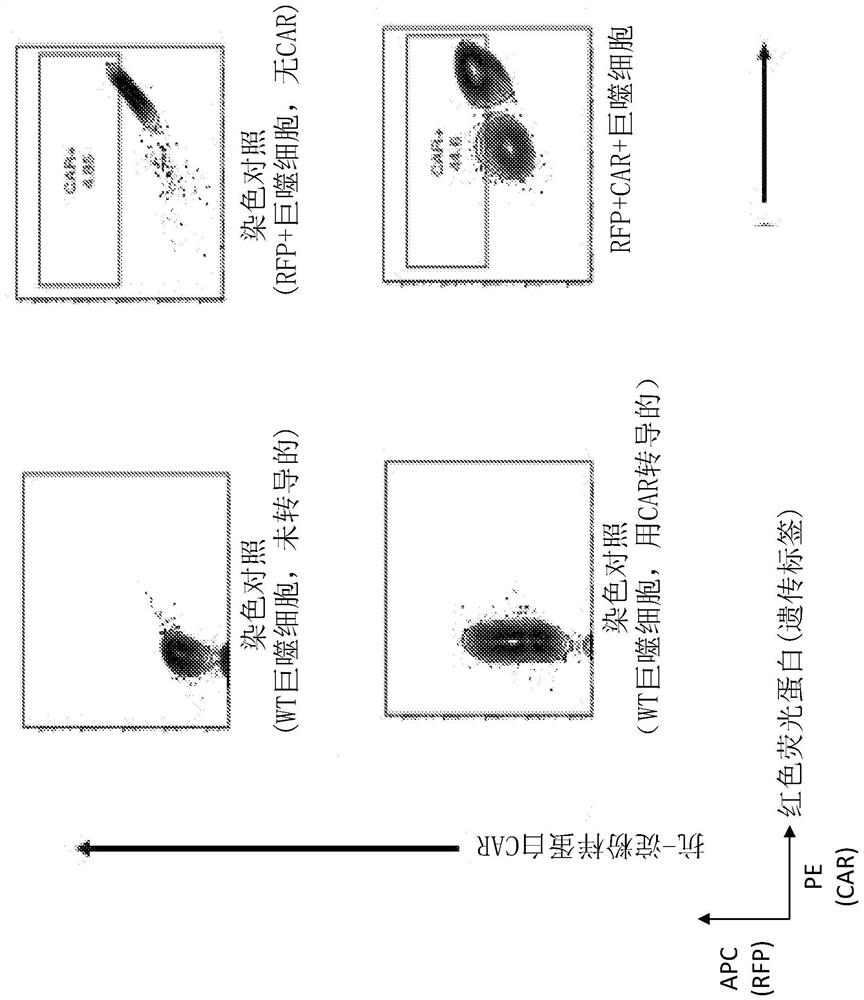
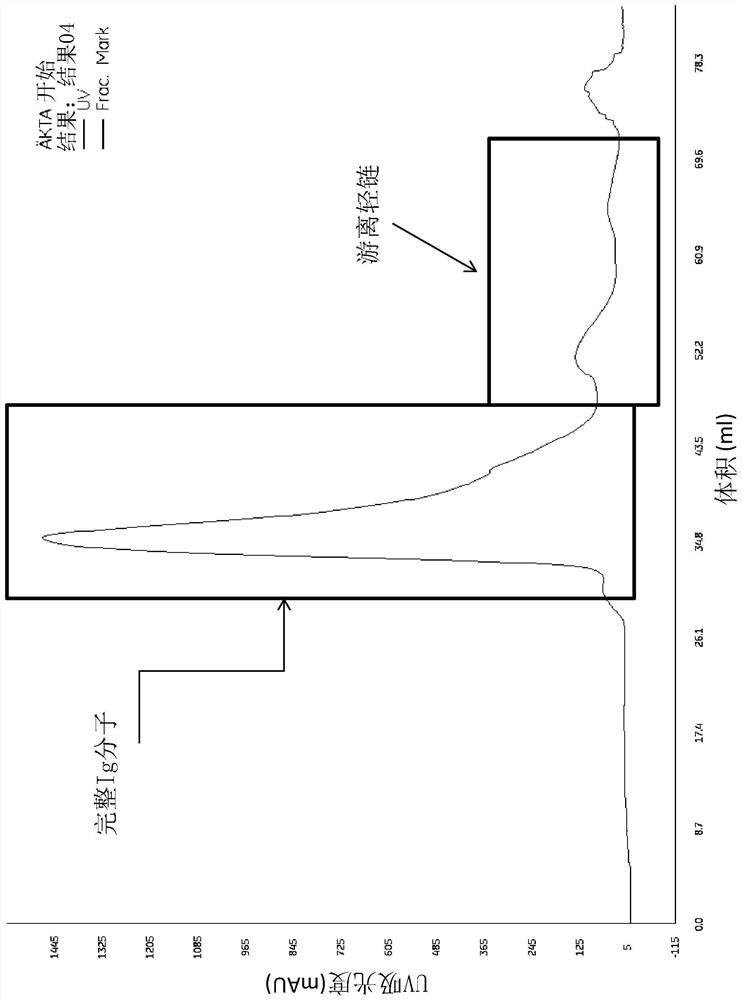
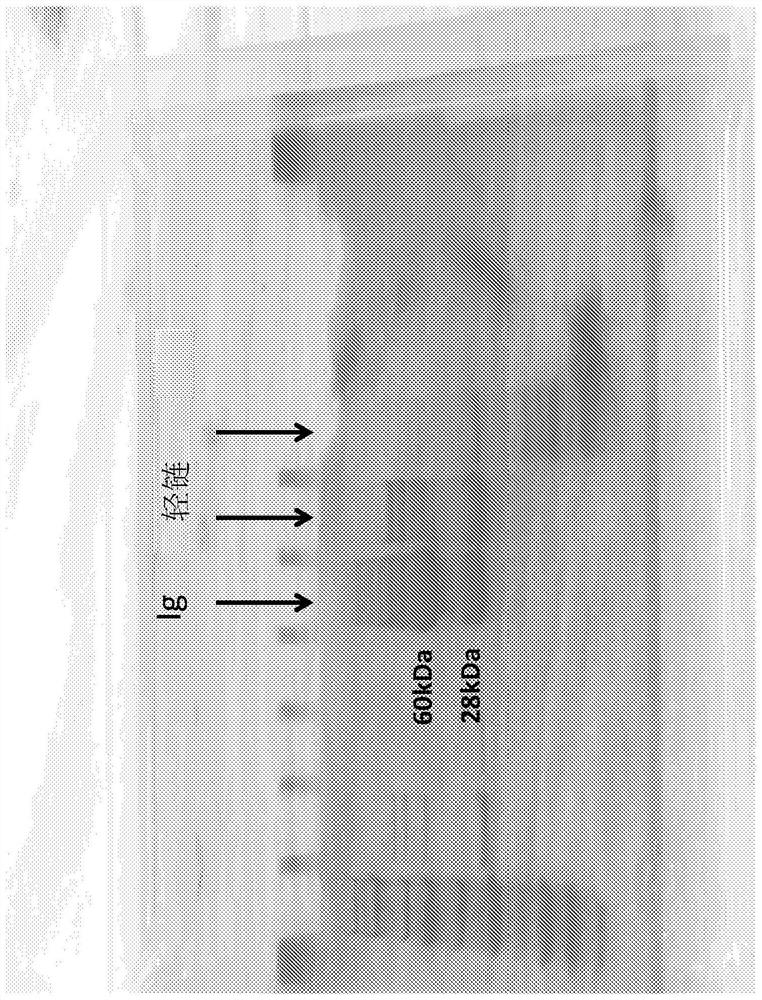
Modified monocytes/macrophages/dendritic cells expressing chimeric antigen receptors and uses in diseases and disorders associated with protein aggregates
A technology of chimeric antigen receptors and monocytes, applied to genetically modified cells, receptors/cell surface antigens/cell surface determinants, animal/human proteins, etc., can solve abnormal collagen protein deposition that does not directly target things and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0296] Example 1: Generation of CAR Genetically Engineered Macrophages Targeting Serum Amyloid P
[0297] By constructing a plasmid comprising the first-generation CAR backbone and the sequence of a commercially available anti-amyloid P antibody, such as anti-serum amyloid P antibody clone EP1018Y (Abcam cat. no. ab45151), clone 14B4 (Abcam cat. no. ab27313), clone 5.4A (Cat. No. CBL304 from Millipore), or any commercially available or proprietary antibody or target recognition portion that binds serum amyloid P to produce a protein containing a protein that recognizes serum amyloid P (SAP) CAR constructs of extracellular domains of scFv of antibodies. NEOD001 scFvs that selectively target neo-epitopes on misfolded antibody light chains can also be used. Additionally, the extracellular domain of the CAR may include synthetic targeting moieties that recognize epitopes on SAP, such as aptamers, darpins, naturally occurring or synthetic SAP receptors, affibodies, or other engine...
Embodiment 2
[0300] Example 2: Elimination or reduction of targets carrying serum amyloid P protein epitopes via phagocytosis of CAR genetically engineered macrophages
[0301] To demonstrate the elimination or reduction of target proteins or protein aggregates in in vivo models, protein xenograft models can be used in which SAP is conjugated to a fluorescent marker with a direct chemical conjugation kit (AF488 or VivoTrack 680) and treated by surgery Implanted into the liver of NSGS mice. After the engraftment period, mice will receive intravenous injections of vehicle alone (phosphate-buffered saline), control non-engineered macrophages, or CAR macrophages directed against SAP (n=5 per group). Injections were repeated every 3 days for 5 cycles. At the end of treatment, mice will be euthanized and liver tissue collected. Whole-mount fluorescence imaging of the entire liver will be performed using IVISSpectrum (Perkin Elmer), and signal intensity will be compared between treatment groups...
Embodiment 3
[0304] Example 3: Generation of CAR Genetically Engineered Macrophages Targeting Amyloid β
[0305] using scFv containing antibodies that recognize amyloid beta, or the tertiary structure of misfolded proteins that make up amyloid plaques (Glabe, 2004. Trends Biochem Sci. 2004 Oct;29(10):542-7), or Oligomer-specific antibodies that recognize the extracellular domain of misfolded proteins that form amyloid plaques (Kayed et al., 2003. Science. Apr 18;300(5618):486-9) generate CAR constructs. By constructing a commercially available anti-amyloid β antibody (such as Abcam of Rockland Antibodies and Assays or ab2539 of antibody number 600-401-253S) or a γ antibody (gammabody) (such as in Perchiacca et al. people, 2012.PNAS 2012January, 109(1)84-89) or many other proprietary or custom-synthesized antibodies that bind to epitopes on the amyloid-beta precursor protein or amyloid aggregates formed Or other plasmids with the sequence of the target recognition part to generate CAR.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com