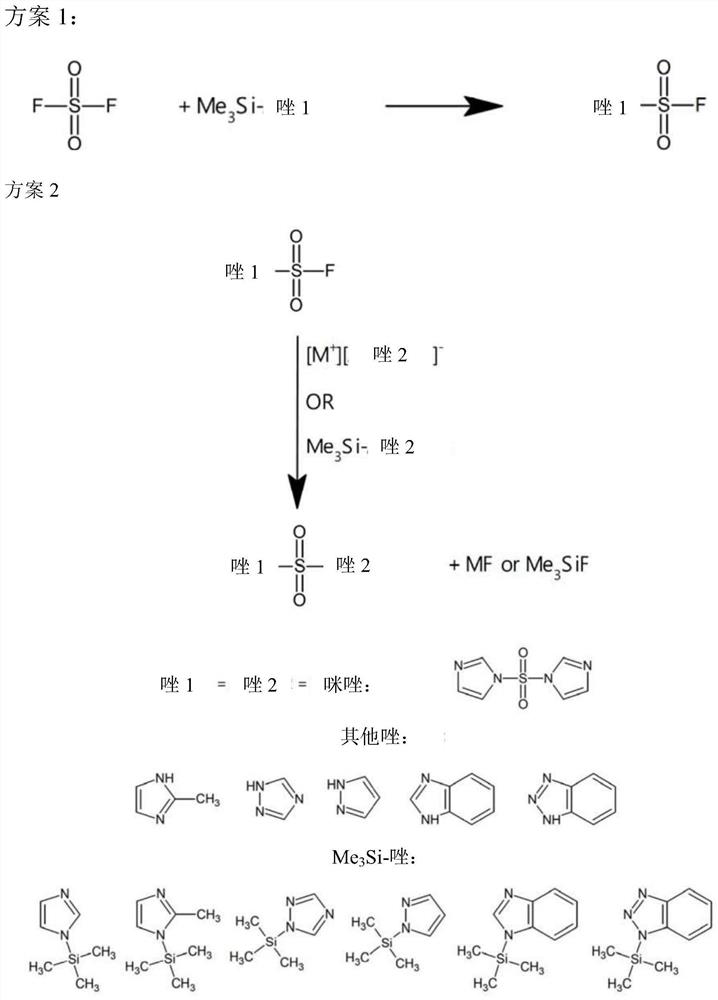
Sulfonyldiazoles and n-(fluorosulfonyl)azoles, and methods of making the same
A technology of sulfonyl diazole and fluorosulfonyl group, which is applied in the field of sulfonyl diazole and N-(fluorosulfonyl) azole and their preparation, and can solve the problems of increased cost of manufacturing process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
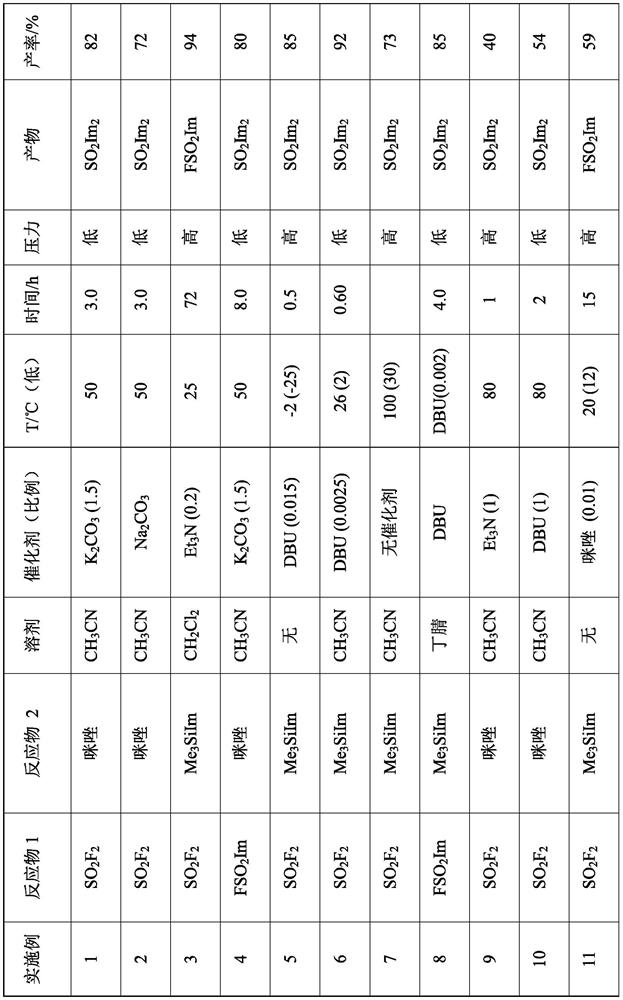
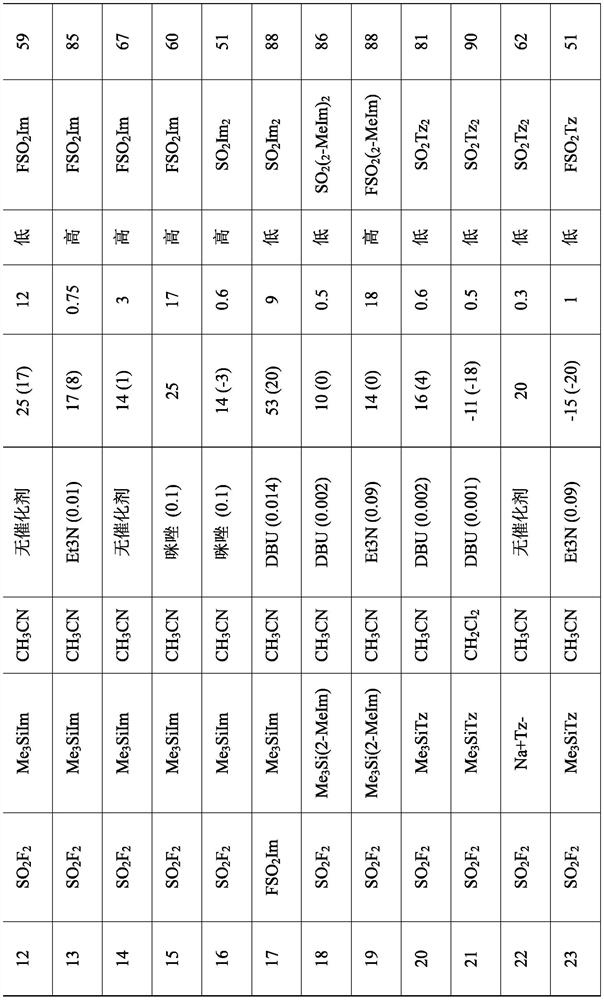
Embodiment 1
[0116] Embodiment 1: Sulfonyldiimidazole (SO 2 Im 2 ). In a 1L 4-neck round bottom flask, charge anhydrous powdered K 2 CO 3 (124g, 0.9mol) and a solution of imidazole in acetonitrile (2mol, 300mL, 0.6mol). The contents of the vessel were stirred magnetically and evacuated to constant static pressure (9.7 kPa). Add SO in 5min at 93kPa 2 f 2 (30.5 g, 0.3 mol), the vessel was warmed from 24°C to 40°C. After stirring for another 36 min, the vessel had dropped to 30°C / 49kPa. Fill the vessel to atmospheric pressure with nitrogen and replace the gas inlet with a mercury bubbler. The progress of the reaction was monitored by GCMS. The vessel was adjusted to 40 °C for 2 h, then to 50 °C for 3 h, after which the reaction was considered complete, although imidazole and FSO 2 Im is still present in the GCMS trace. Filter the contents of the vessel and wash the solids with copious amounts of warm acetonitrile. The combined filtrates were concentrated to dryness to give a solid...
Embodiment 2
[0117] Example 2: Sulfonyldiimidazole. Add anhydrous powdered Na to a 1 L 4-neck round bottom flask 2 CO 3 (128g, 1.2mol) and a solution of imidazole in acetonitrile (2mol, 300mL, 0.6mol). The vessel was placed in an ambient water bath, the vessel contents were stirred magnetically, and evacuated to a constant static pressure (11 kPa). Add SO within 42min at 93kPa, 24°C 2 f 2 (30 g, 0.3 mol), and after stirring for a further 73 min, the vessel was lowered to 23°C / 25kPa. Fill the vessel to atmospheric pressure with nitrogen and replace the gas inlet with a mercury bubbler. The progress of the reaction was monitored by GCMS. The vessel was adjusted to 38-40°C for 12h, then to 50°C for 3h, after which the reaction was considered complete, although imidazole and FSO 2 Im is still present in the GCMS trace. Filter the contents of the vessel and wash the solids with warm acetonitrile (300 mL). The combined filtrates were concentrated to dryness to give a solid which was disso...
Embodiment 3
[0118] Example 3: 1H-imidazole-1-sulfonyl fluoride (FSO 2 Im). Add 1-trimethylsilyl-1H-imidazole (Me 3 SiIm, 151g, 1.1mol) and triethylamine (30mL), sealed, ice-cooled, and evacuated to a constant static pressure (2.9kPa). Rapid addition of SO under pressure 2 f 2 (123 g, 1.21 mol). Then the stirred tank was kept at 25° C. for 3 days, and the pressure of the tank decreased from 1275 kPa to 212 kPa. The vessel was stirred for an additional day, maintaining a pressure of around 212 kPa. The vessel was then vented, purged with nitrogen, and the vessel contents distilled at 56 °C / 3.3 kPa to give the product FSO 2 Im (153g, 1.02mol, 94%) FSO 2 Im is easy to be supercooled, with a melting point of 31.5°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap