Safe and non-toxic nerve cell restoring drug
A nerve cell, safe and non-toxic technology, applied in the field of nerve cell repair drugs, can solve problems such as increased absorption and reduced drug dosage, and achieves high cure rate, guaranteed barrier breaking rate, and good drug effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
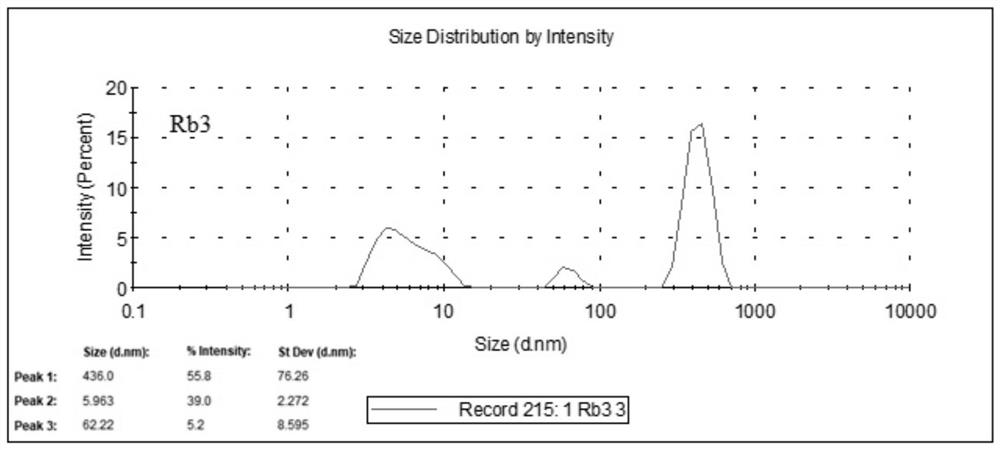
[0022] see figure 1 , in the safe and non-toxic nerve cell repair medicine disclosed in the preferred embodiment 1 of the present application, ginsenoside Rb3 is used as the main component, and the main component of ginsenoside Rb3 contains 55.8 parts of coarse-grained Rb3 particles and 5.2 parts of medium-sized Rb3 particles , 39 parts of fine-grained Rb3 particles. Among them: the particle size of coarse-grained Rb3 particles is not less than 100nm, the particle size of medium-sized Rb3 particles is between 10nm and 100nm, and the particle size of fine-grained Rb3 particles is not more than 10nm. In addition, the particle diameters of the coarse-grained Rb3 particles are concentrated at 436 nm, and the particle diameters of the fine-grained Rb3 particles are concentrated at 6 nm.
Embodiment 2
[0024] In the safe and non-toxic nerve cell repair drug disclosed in the preferred embodiment 2 of the present application, ginsenoside Rb3 is used as the main component, and the main component of ginsenoside Rb3 contains 58 parts of coarse-grained Rb3 particles and 5.5 parts of medium-sized Rb3 particles. 40 parts of fine-grained Rb3 particles. Among them: the particle size of coarse-grained Rb3 particles is not less than 100nm, the particle size of medium-sized Rb3 particles is between 10nm and 100nm, and the particle size of fine-grained Rb3 particles is not more than 10nm. In addition, the particle diameters of the coarse-grained Rb3 particles are concentrated at 436 nm, and the particle diameters of the fine-grained Rb3 particles are concentrated at 6 nm.
Embodiment 3
[0026] In the safe and non-toxic nerve cell repair drug disclosed in Preferred Example 3 of the present application, ginsenoside Rb3 is used as the main component, and the main component of ginsenoside Rb3 contains 55 parts of coarse-grained Rb3 particles and 5 parts of medium-sized Rb3 particles. 38 parts of fine-grained Rb3 particles. Among them: the particle size of coarse-grained Rb3 particles is not less than 100nm, the particle size of medium-sized Rb3 particles is between 10nm and 100nm, and the particle size of fine-grained Rb3 particles is not more than 10nm. In addition, the particle diameters of the coarse-grained Rb3 particles are concentrated at 436 nm, and the particle diameters of the fine-grained Rb3 particles are concentrated at 6 nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com