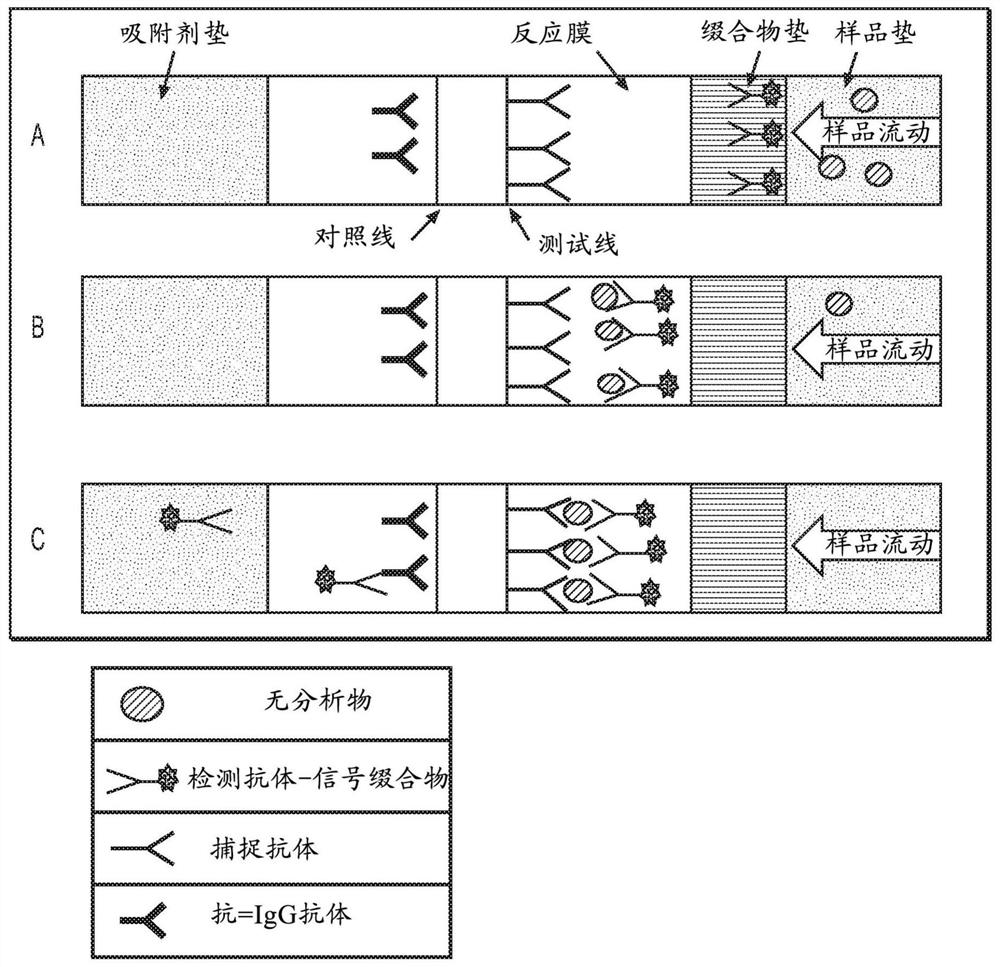
Antibody pairs for use in a rapid influenza b diagnostic test
A type of influenza B and antibody pair technology, applied in biological testing, disease diagnosis, measuring devices, etc., can solve problems such as unavailable combination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1: Testing of Half (ie "wet") Dip Tablet Dose Response of Antibody Conjugates to NP Antigen of Influenza B (Florida / 4 / 2006).
[0128] Capture antibodies were striped onto 2.5 x 30 cm nitrocellulose membrane cards at a rate of 0.1 µl / sec using the Isoflow reagent dispensing module. The cards were dried overnight at ambient temperature in a humidity controlled drying room and then laminated with a 21 mm wide cellulose fiber core (222 Ahlstrom film). The cards were cut into 5 mm wide strips using a motion cutter (KinematicMatrix 2360), resulting in 5 mm wide semi-lateral flow impregnated sheets ("semi-dipped sheets").
[0129] 20 μl each of the NP stock solution and the detection antibody-gold conjugate suspension were added to the 96-well plate and incubated for 5 minutes. Place the half-dipped sample in the well and let stand until all the liquid is absorbed.
[0130] exist Figure 4 Shown above are the results of visual scoring of sample test line color inten...
Embodiment 2
[0132] Example 2: Evaluation of antibody pairs in "dry-up" format (F).
[0133] Nitrocellulose was stuck into strips with 1.0 mg / ml capture antibody and dried overnight at ambient temperature in a humidity-controlled environment. Spray the conjugate pad on the membrane card with detection antibody colloidal gold conjugate.
[0134] The apparatus was run for 20 min with 60 μl of each NP standard using standard techniques.
[0135] Figure 5 The results of visually scoring the test lines relative to the Rann scale as previously described are shown in .
[0136] Antibody pair (F), GenWay GWB-T00595 (capture) and Fitzgerald-fiil0-I55P (B) (conjugate) is shown to detect NP antigen of influenza B (Florida / 4 / 2006) down to 1 ng / ml.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com