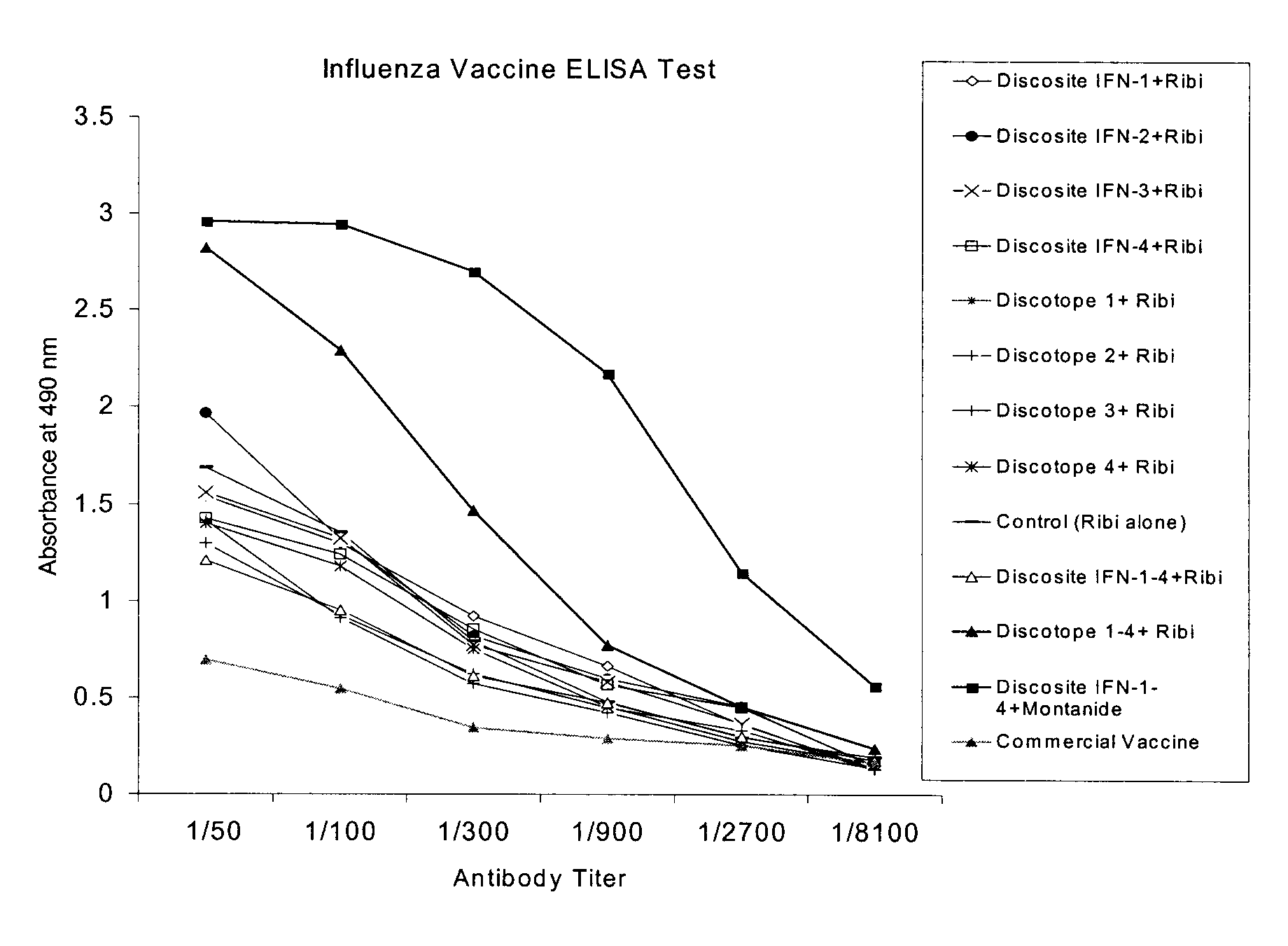
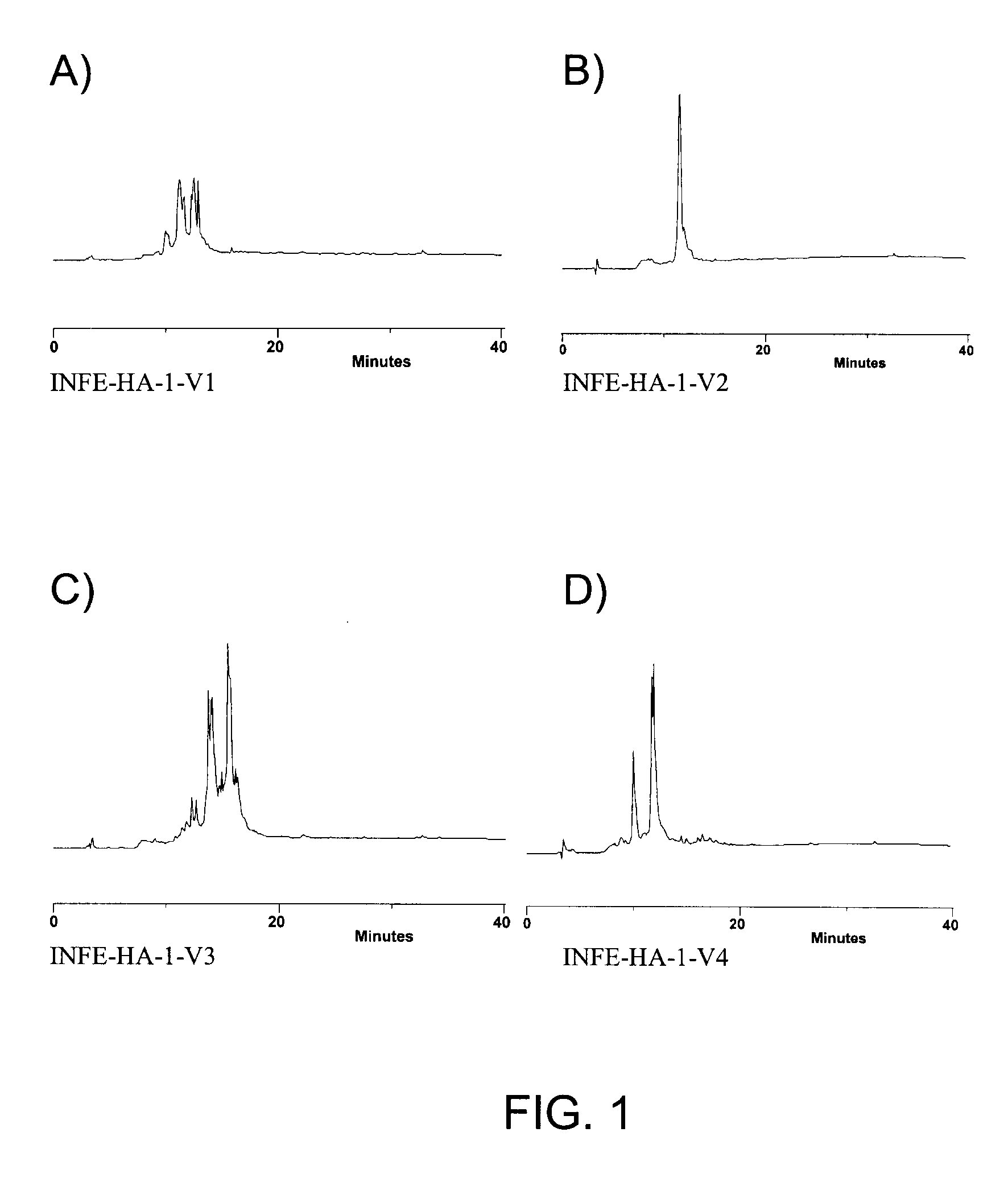
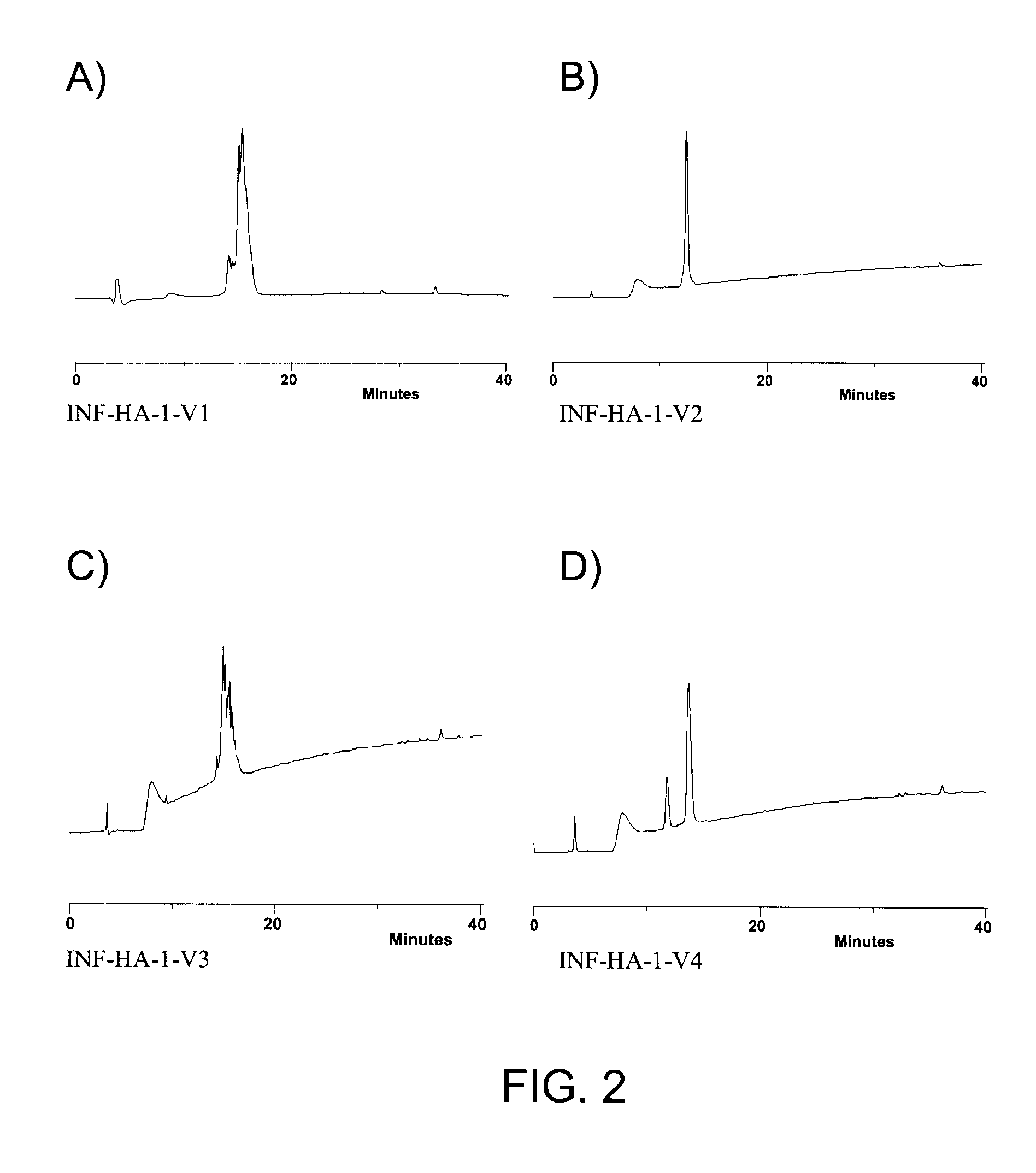
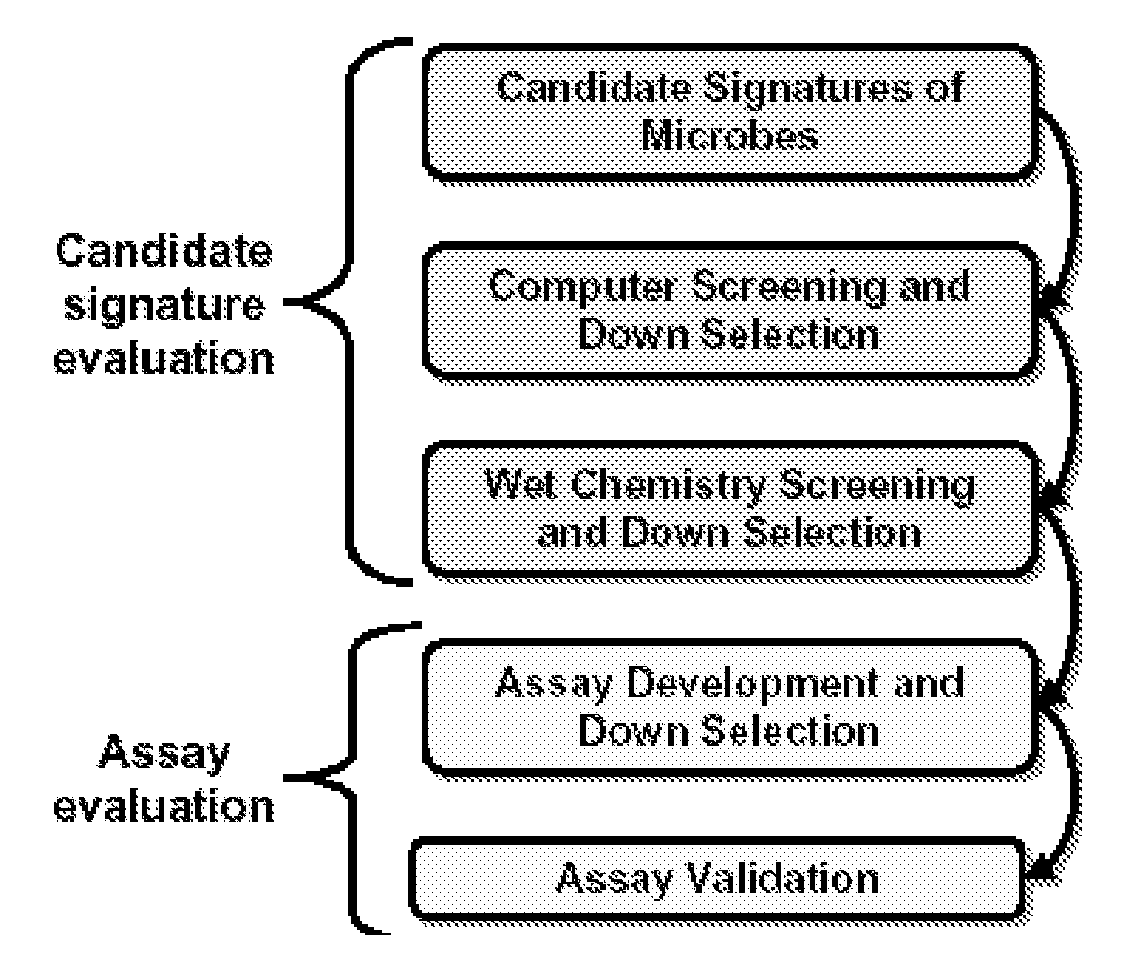
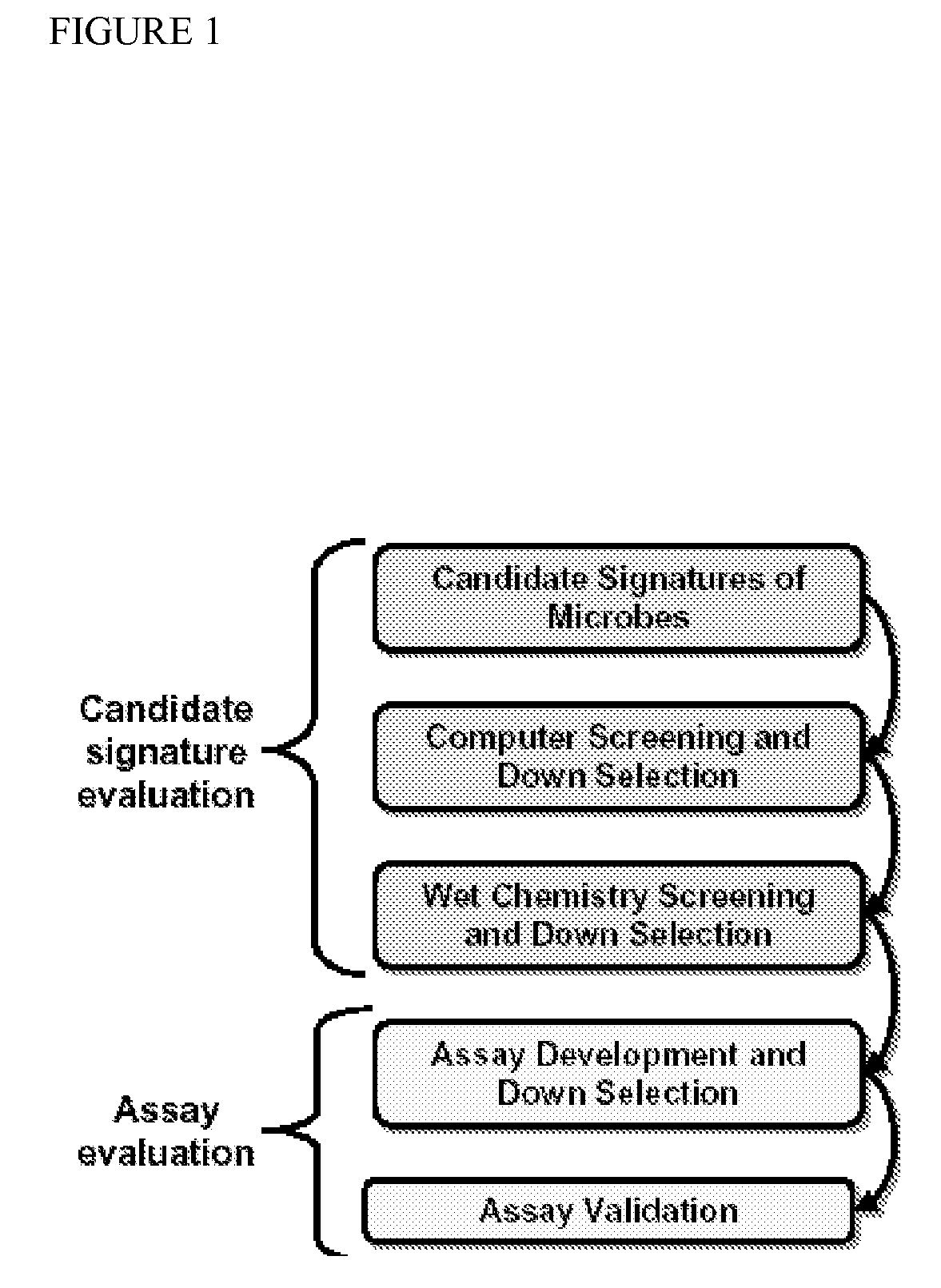
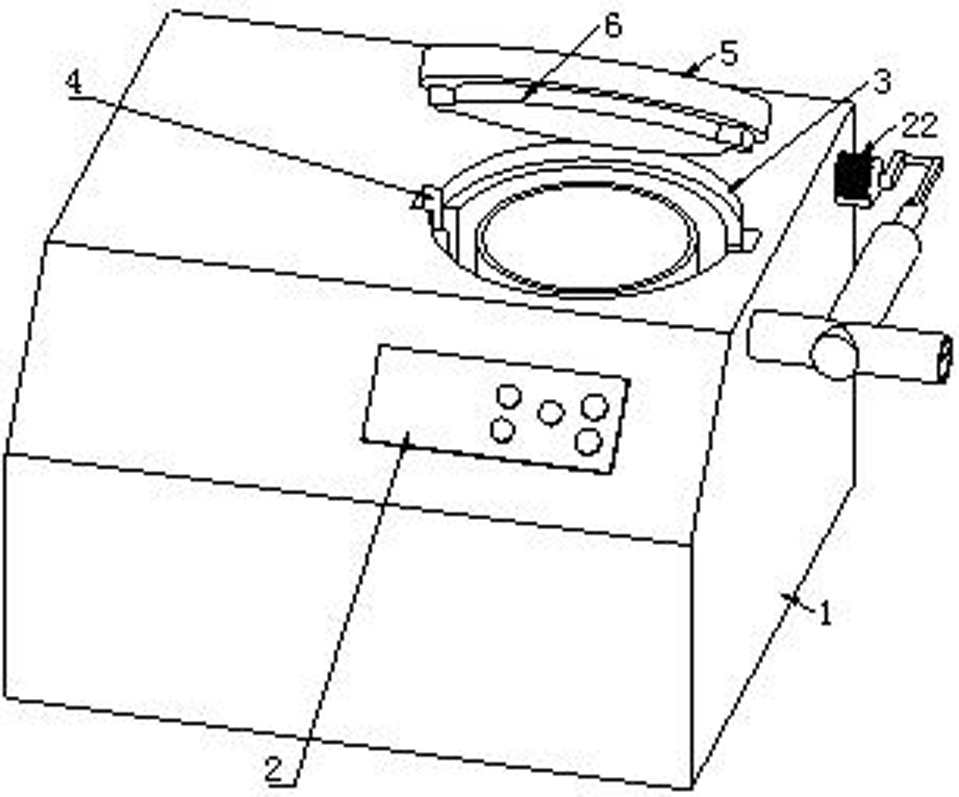
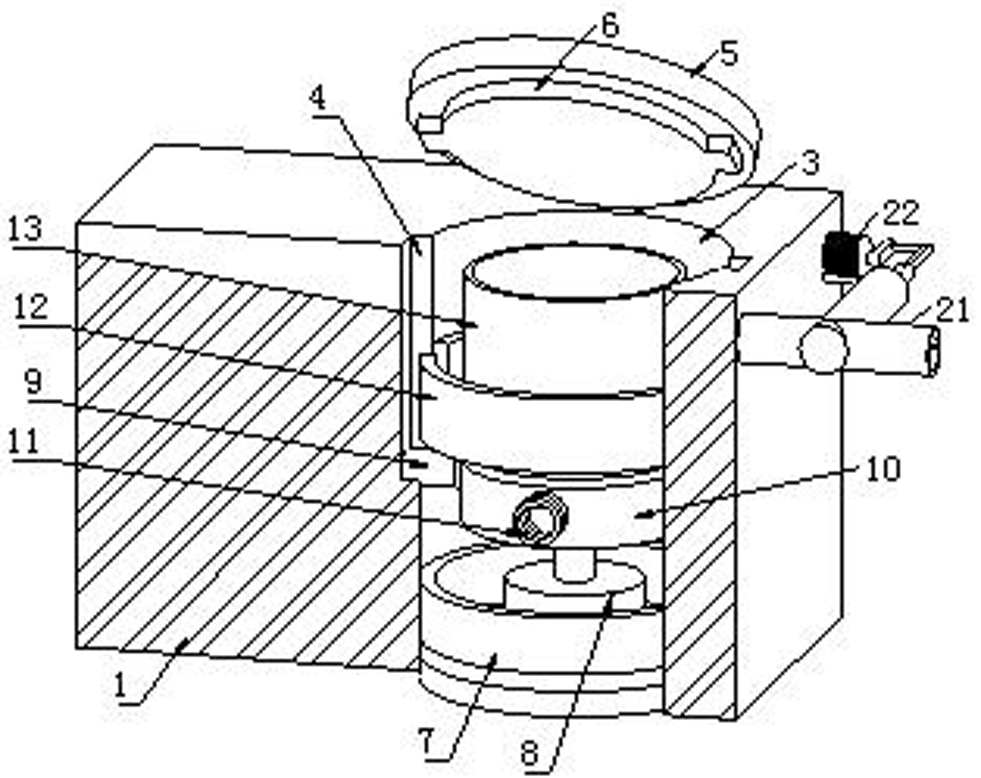
Patents
Literature
30 results about "H influenzae type b" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Meningitis - H. influenzae. Meningitis is an infection of the membranes covering the brain and spinal cord. This covering is called the meninges. Bacteria are one type of germ that can cause meningitis. Haemophilus influenzae type b is one kind of bacteria that causes meningitis.
Copper ion compositions and methods of treatment for conditions caused by coronavirus and influenza
Provided herein are formulations containing copper ions and methods of treating underlying infections and conditions caused by coronavirus, particularly COVID-19, and influenzas, particularly influenza A and / or influenza B using such formulations. Methods of treating the underlying viruses and their resultant conditions using topical copper ion treatments are provided. A topical treatment in its basic form comprises a biocompatible copper ion solution or suspension obtained by leaching of the copper ions from copper metal. The copper ion solution or suspension may be combined with various carriers to form the copper ion treatment including creams or solutions. Methods of making the copper ion solution or suspension from solid copper metal in a biocompatible solution are also provided.
Owner:CDA RES GROUP
Peptide-Based Influenza Vaccine Formulation
InactiveUS20090104216A1Improve responseBroad protectionSsRNA viruses negative-sensePeptide/protein ingredientsEpitopeInfluenza vaccine
Peptide-based anti-influenza formulations against influenza A and B are disclosed. The peptides are derived from influenza-based epitopes. The formulations are based on peptide mixtures which may be formulated so that variability is present at particular residues. The formulations can be used to prepare vaccines for preventing influenza in human, avian, murine or equine animals.
Owner:VARIATION BIOTECHNOLOGIES INC
Multiplex detection of respiratory pathogens
ActiveUS20090305229A1Sugar derivativesMicrobiological testing/measurementViral typeRespiratory pathogens
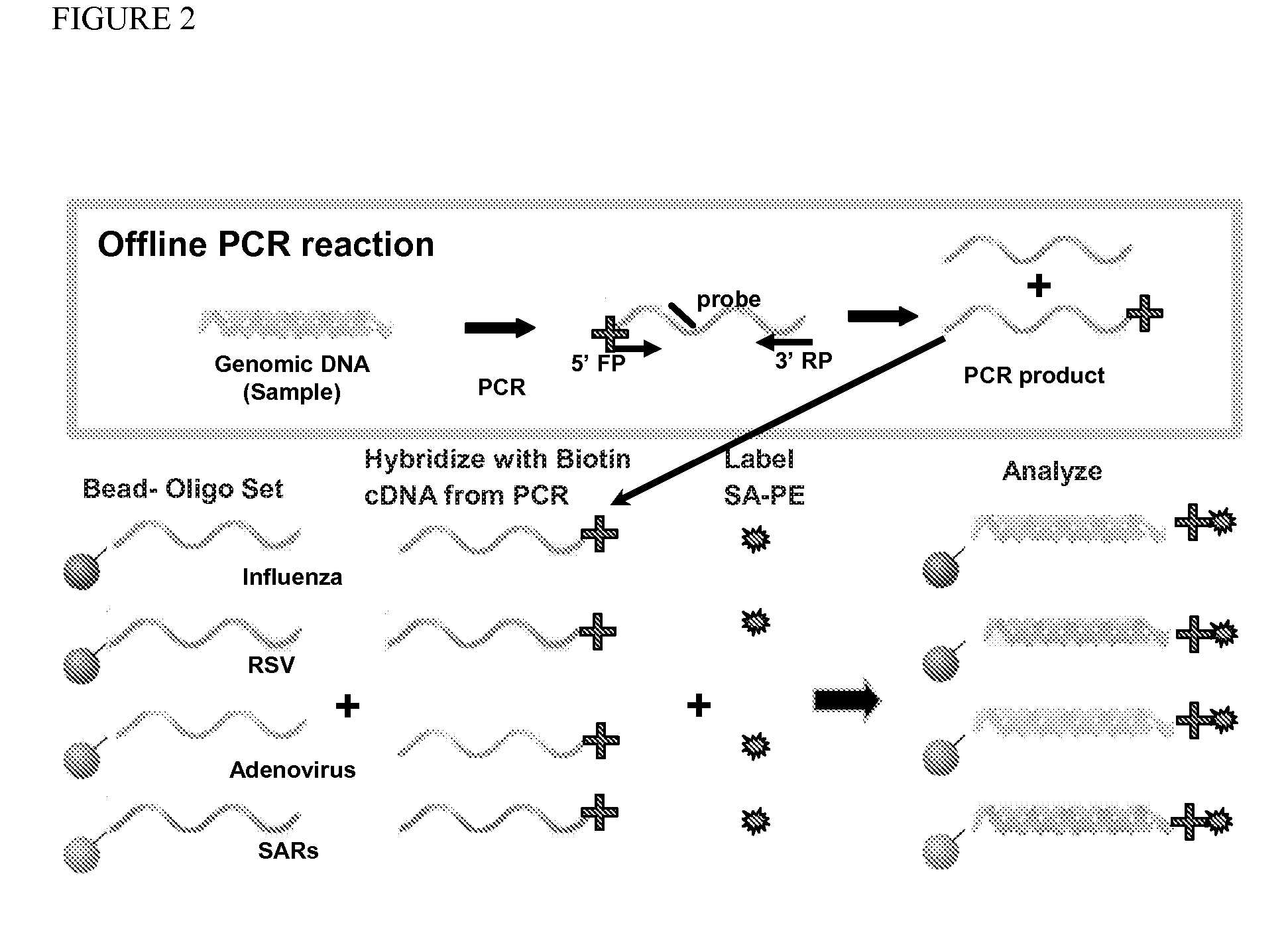
Described are kits and methods useful for detection of respiratory pathogens (influenza A (including subtyping capability for H1, H3, H5 and H7 subtypes) influenza B, parainfluenza (type 2), respiratory syncytial virus, and adenovirus) in a sample. Genomic sequence information from the respiratory pathogens was analyzed to identify signature sequences, e.g., polynucleotide sequences useful for confirming the presence or absence of a pathogen in a sample. Primer and probe sets were designed and optimized for use in a PCR based, multiplexed Luminex assay to successfully identify the presence or absence of pathogens in a sample.
Owner:LAWRENCE LIVERMORE NAT SECURITY LLC
Detection of influenza virus
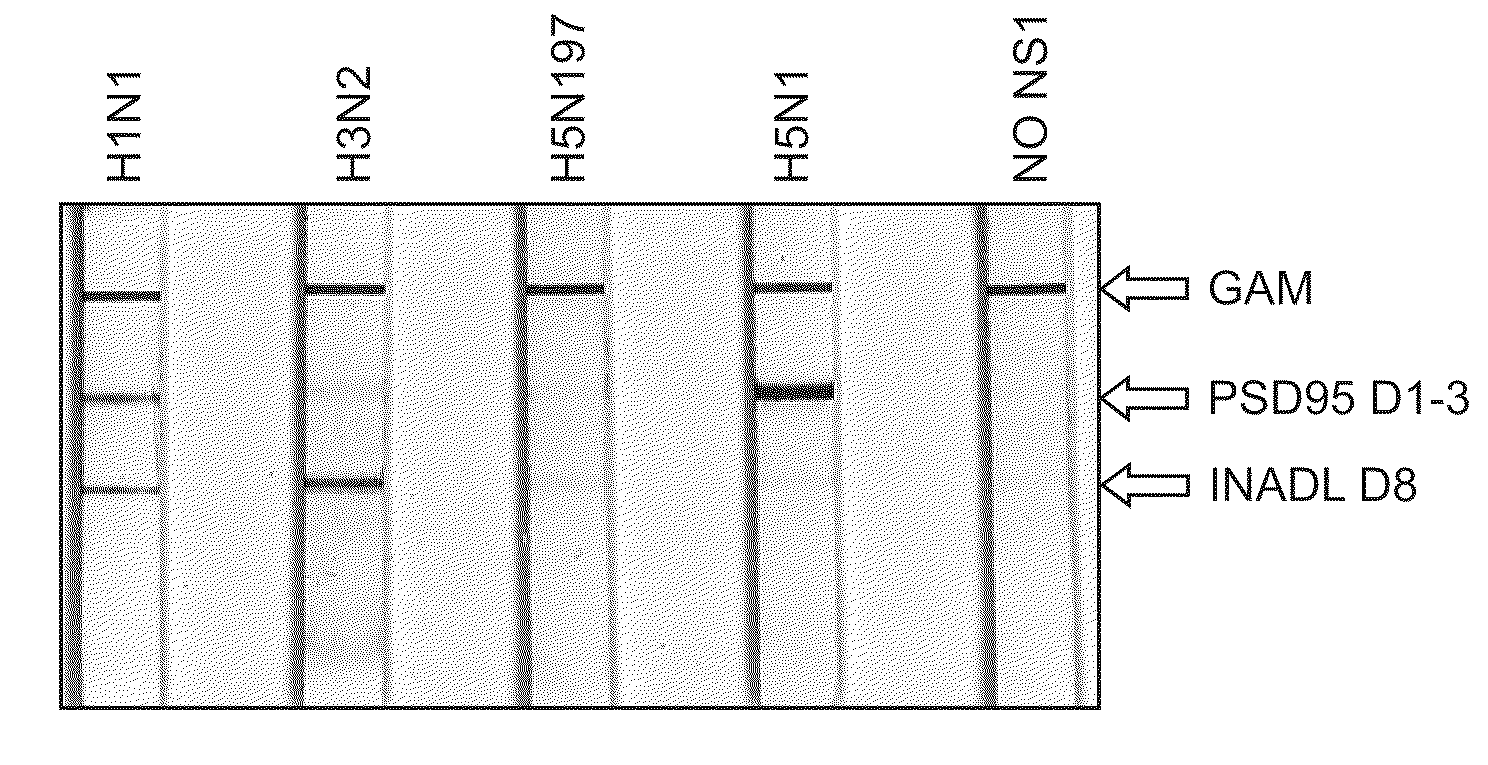
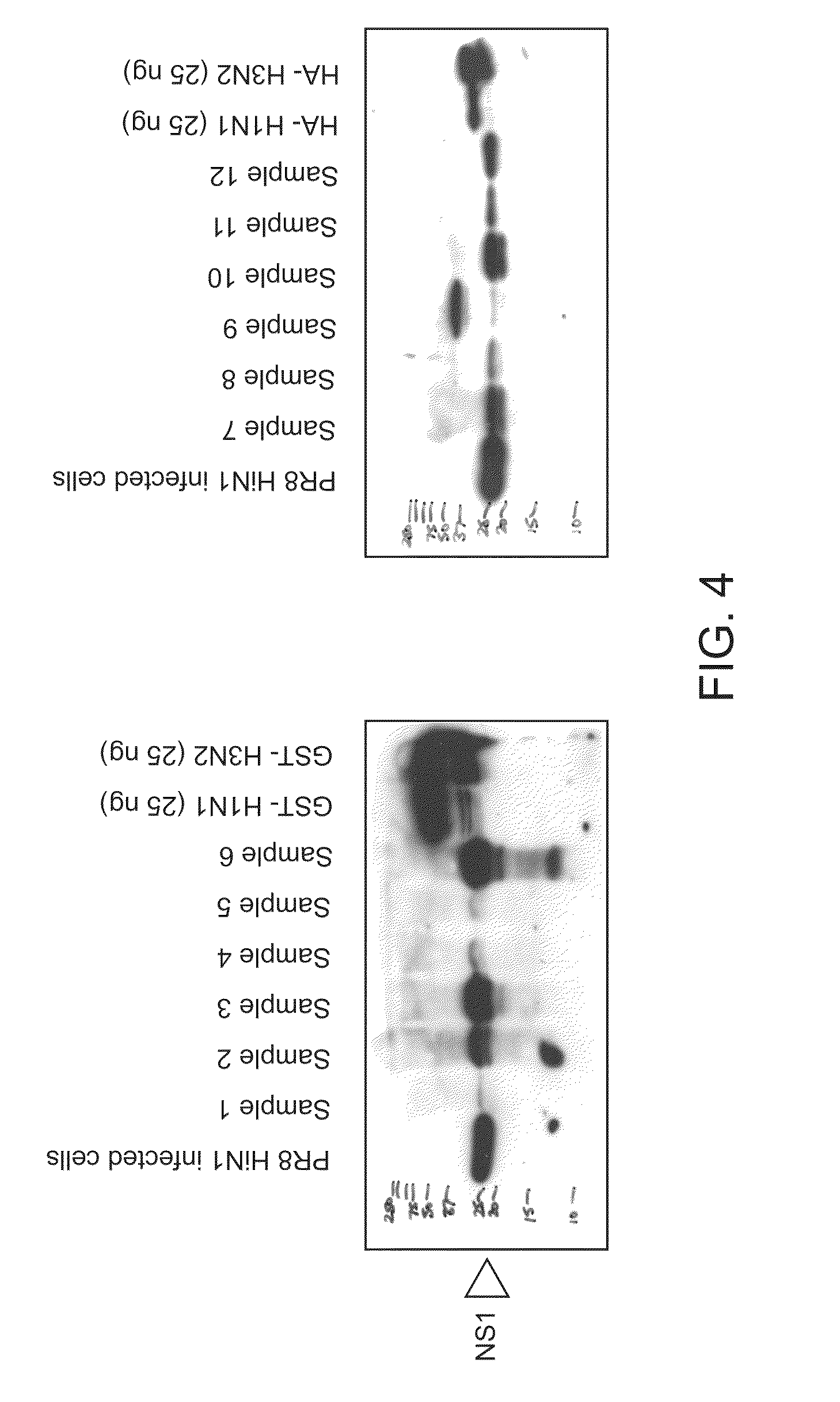
The present application describes methods for detecting influenza A and / or influenza B and / or distinguishing between pathogenic and seasonal influenza A subtypes. Many of these preferred formats employ pan-specific antibodies (i.e., that react with all or at least multiple strains within an influenza type) to detect presence of influenza A and / or influenza B and PDZ domains in combination with panspecific antibodies to influenza A to distinguish pathogenic and seasonal influenza A subtypes.
Owner:AVC ROYALTY FUND I
Copper ion compositions and methods of treatment for conditions caused by coronavirus and influenza
Provided herein are formulations containing copper ions and methods of treating underlying infections and conditions caused by coronavirus, particularly COVID-19, and influenzas, particularly influenza A and / or influenza B using such formulations. Methods of treating the underlying viruses and their resultant conditions using topical copper ion treatments are provided. A topical treatment in its basic form comprises a biocompatible copper ion solution or suspension obtained by leaching of the copper ions from copper metal. The copper ion solution or suspension may be combined with various carriers to form the copper ion treatment including creams or solutions. Methods of making the copper ion solution or suspension from solid copper metal in a biocompatible solution are also provided.
Owner:CDA RES GROUP
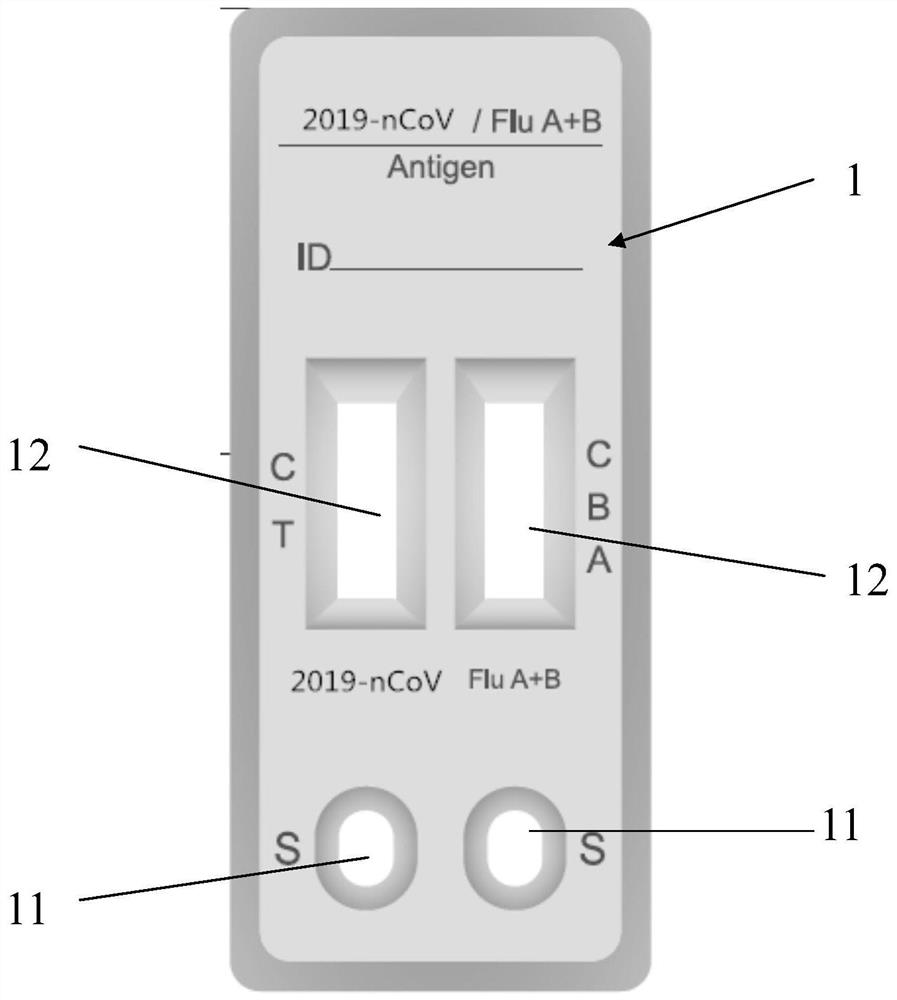
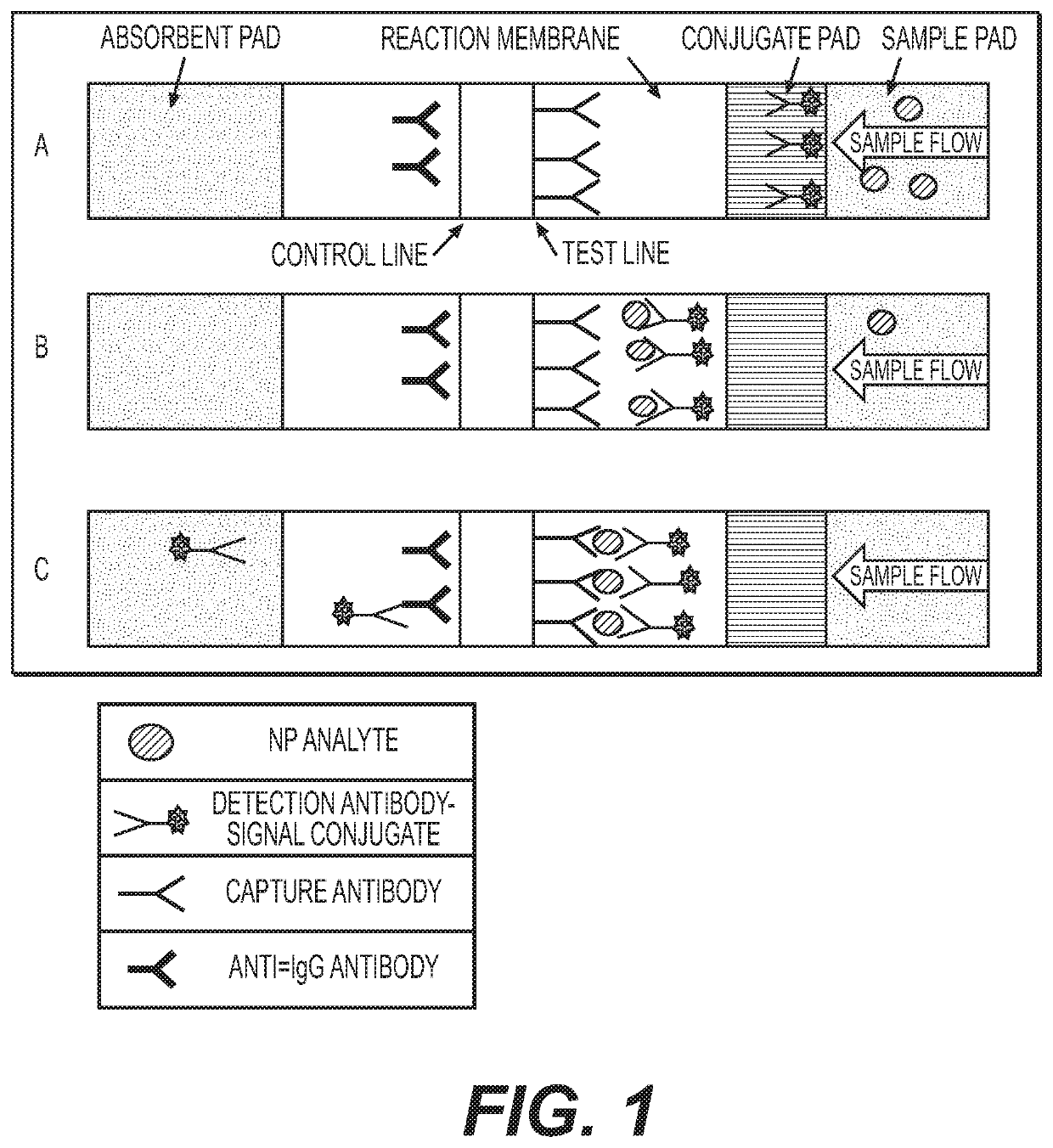
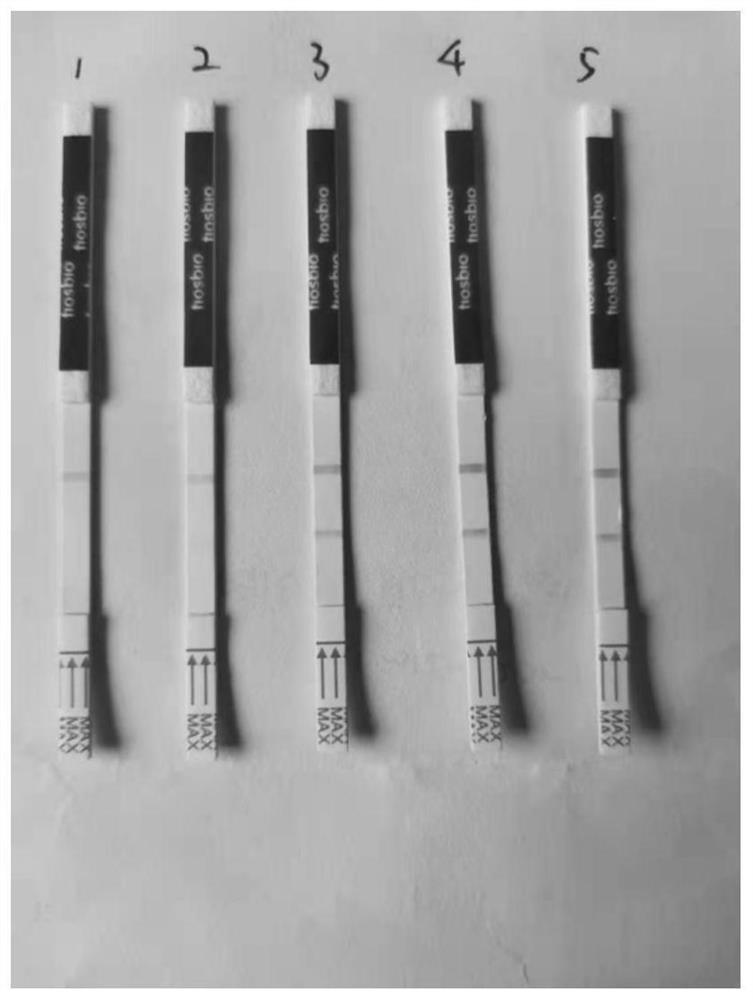
Joint detection kit for novel influenza virus and influenza virus A/B as well as preparation method and application of joint detection kit
ActiveCN112904001AReduce the risk of transmissionShort detection timeBiological testingImmunoassaysAntigenViral antibody
The invention discloses a joint detection kit for novel influenza virus and influenza virus A / B as well as preparation method and application of the joint detection kit. The joint detection kit comprises a detection test strip, the detection test strip comprises a sample pad, a combination pad, a detection pad and an absorption pad which are sequentially connected in an overlapped mode, and the detection pad is provided with a detection line and a quality control line which are parallel to each other; the detection line comprises a first detection line, a second detection line and a third detection line which are separated from one another, the first detection line is coated with a new coronavirus antibody, the second detection line is coated with an influenza A virus antibody, and the third detection line is coated with an influenza B virus antibody. The joint detection kit provided by the invention can simultaneously detect three items of the new coronavirus, the influenza A and the influenza B after one-time sampling treatment, is short in detection time and simple to operate, and avoids missed diagnosis or misdiagnosis in single item detection; besides, the repeated contact between medical staff and patients in multiple sample collection processes is reduced, and the risk of epidemic situation spreading is reduced.
Owner:厦门市波生生物技术有限公司
AAV mediated influenza vaccines
A non-replicating recombinant adeno-associated associated virus (rAAV) having an AAV capsid having packaged therein a vector genome which comprises AAV inverted terminal repeat sequences and at least one nucleic acid sequence encoding four different immunoglobulin regions (a), (b), (c) and (d) is provided. The rAAV-expressed immunoglobulins are useful for providing passive immunization against influenza A and influenza B. Also described herein are compositions containing the rAAV. Methods of vaccinating patients against influenza are provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
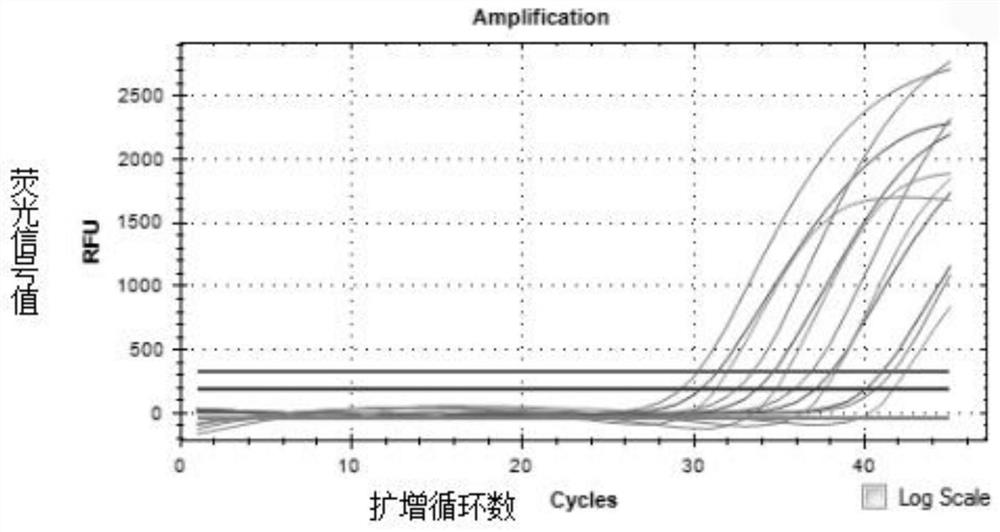
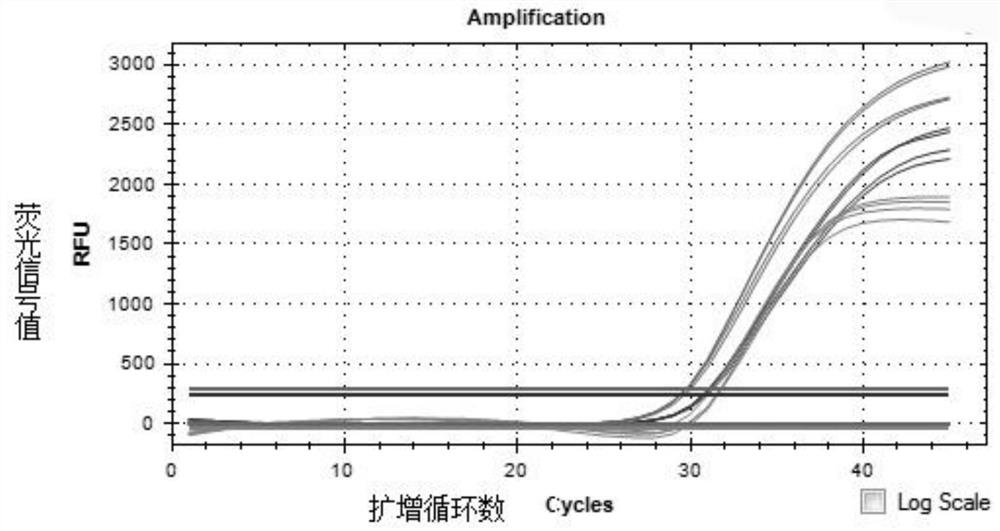
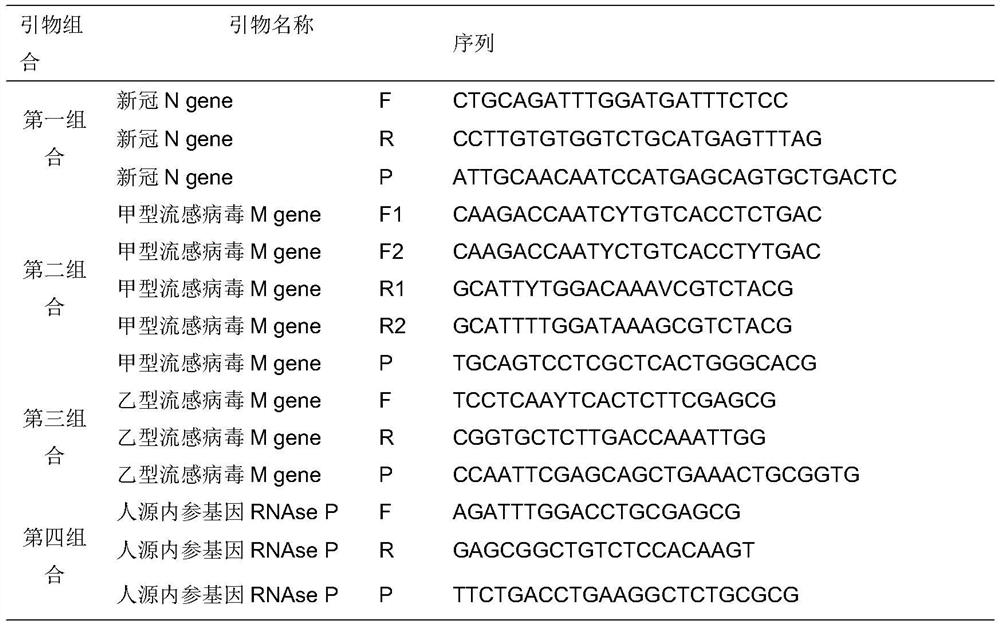
Fully-premixed freeze-drying multi-fluorescent PCR detection kit for novel coronavirus, influenza A virus and influenza B virus and detection method thereof
PendingCN112760415ALow level of operation requiredQuick filterMicrobiological testing/measurementAgainst vector-borne diseasesFreeze-dryingRespiratory pathogen
The invention discloses a multi-fluorescent PCR rapid detection kit for novel coronavirus, influenza A virus and influenza B virus. The kit comprises freeze-dried solid RT-PCR Mix, liquid redissolution Buffer, freeze-dried solid positive control and freeze-dried solid negative control, wherein the freeze-dried solid RT-PCR Mix contains a primer group corresponding to primer sequences of a SARS-CoV-2 specific gene ORF1ab, an influenza A M gene, an influenza B M gene and a human reference gene RNAse P. The kit combines a multi-fluorescent quantitative PCR technology and a freeze-drying process, utilizes three pairs of special primers and human reference genes to amplify specific sequences of three pathogens in vitro, and performs real-time detection in combination with a fluorescent probe. The detection method is simple and convenient to operate, has low requirements for the operation level of detection personnel, and can detect three common respiratory pathogens at a time, the detection time and the detection cost are greatly saved, rapid screening of large-batch samples is realized, the whole detection process only takes 40 minutes to 1 hours, and results are accurate and reliable.
Owner:青岛巴特菲科技发展有限公司
Application of traditional Chinese medicine composition to preparation of medicament for treating influenza B virus
The invention discloses application of a traditional Chinese medicine composition to preparation of medicaments for treating influenza B virus. The traditional Chinese medicine composition comprises medicines of fructus forsythia, ephedra stem and bitter apricot kernel, and is proved to be capable of confronting influenza B virus effectively; and the traditional Chinese medicine composition has effect on influenza virus substantially better than on other viruses.
Owner:BEIJING YILING PHARMA
COMPOSITIONS AND METHODS FOR THE SIMULTANEOUS DETECTION OF INFLUENZA A, INFLUENZA B, AND SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2 (SARS-CoV-2)
PendingUS20220042117A1Enlarge regionMinimizing the potential for cross-contaminationMicrobiological testing/measurementInfluenza A antigenCare setting
Methods for the rapid detection of the presence or absence of SARS-CoV-2 in biological or non-biological samples are described. These methods are adapted to be performed rapidly in a point-of-care setting. The methods can include performing an amplifying step, a hybridizing step, and a detecting step. Specifically, primers and probes targeting SARS-CoV-2 are provided that are designed for the detection of this target. Additionally, kits and reaction vessels containing primers and probes targeting SARS-CoV-2 are provided. Additionally, methods, kits and reaction vessels for the simultaneous rapid detection of the presence or absence of SARS-CoV-2, influenza A, and influenza B in biological or non-biological samples are described.
Owner:ROCHE MOLECULAR SYST INC
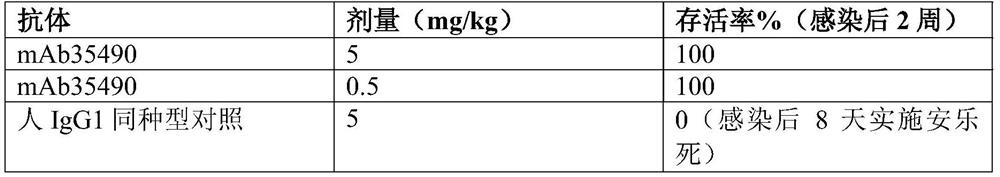
Human monoclonal antibodies broadly protective against influenza B virus and methods of using the same
InactiveUS9181328B2Showed therapeutic efficacyBiological material analysisImmunoglobulins against virusesAntigenAntigen Binding Fragment
Materials and methods are provided for treating influenza B infections in humans. Anti-human influenza virus monoclonal antibodies and antigen-binding fragments thereof having a neutralization activity against a human influenza B virus are provided. Methods for producing anti-human influenza B virus monoclonal antibodies are also provided. The antibodies and antigen-binding fragments thereof can be effective against a wide range of influenza B viral strains. Methods of inhibiting or treating a human influenza B infection are provided. The anti-influenza B therapeutics can also be used to manufacture medicaments effective against influenza B infections, to detect human influenza B in a human subject, for use in pharmaceutical compositions, and for use in kits for at least one of the prevention, the treatment, and the detection of human influenza B in a human subject.
Owner:OSAKA UNIV +3
Human neutralizing antibodies binding to influenza B neuraminidase
ActiveUS10703803B2Immunoglobulins against virusesCarrier-bound/immobilised peptidesAntigenNeuraminidase
The present invention provides influenza neuraminidase (NA)-binding human antibodies, which are capable of specifically binding to and neutralizing at least one influenza B virus strain from the B / Victoria lineage and / or at least one influenza B virus strain from the B / Yamagata lineage, as well as antigen-binding fragment thereof. The invention furthermore relates to the use of said antibodies or antigen-binding fragments in the diagnosis, prophylaxis and / or treatment of influenza infection.
Owner:JANSSEN VACCINES & PREVENTION BV
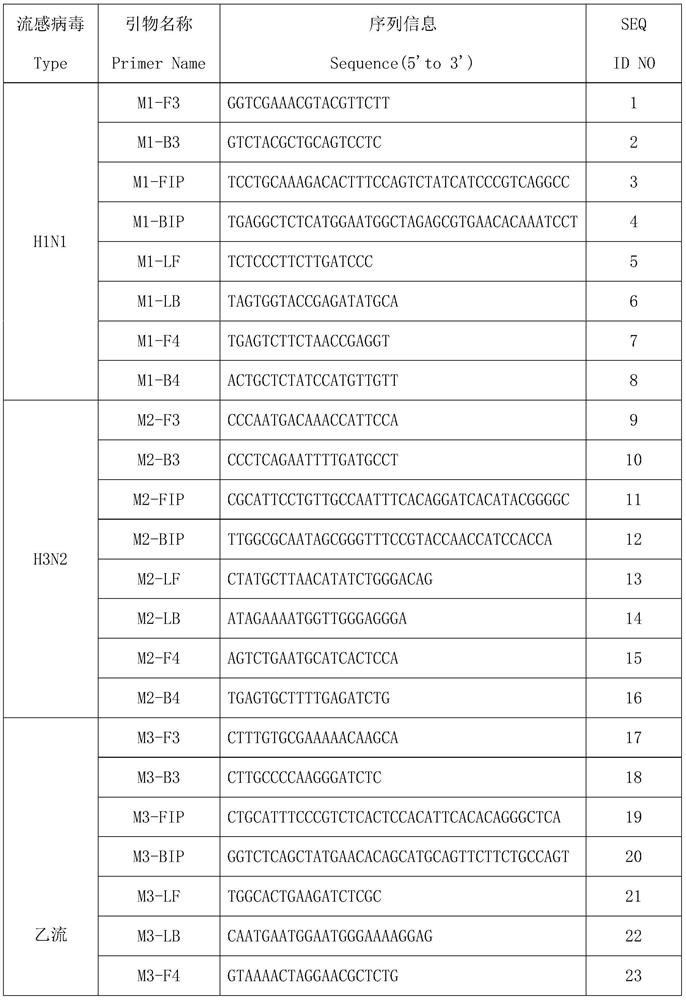
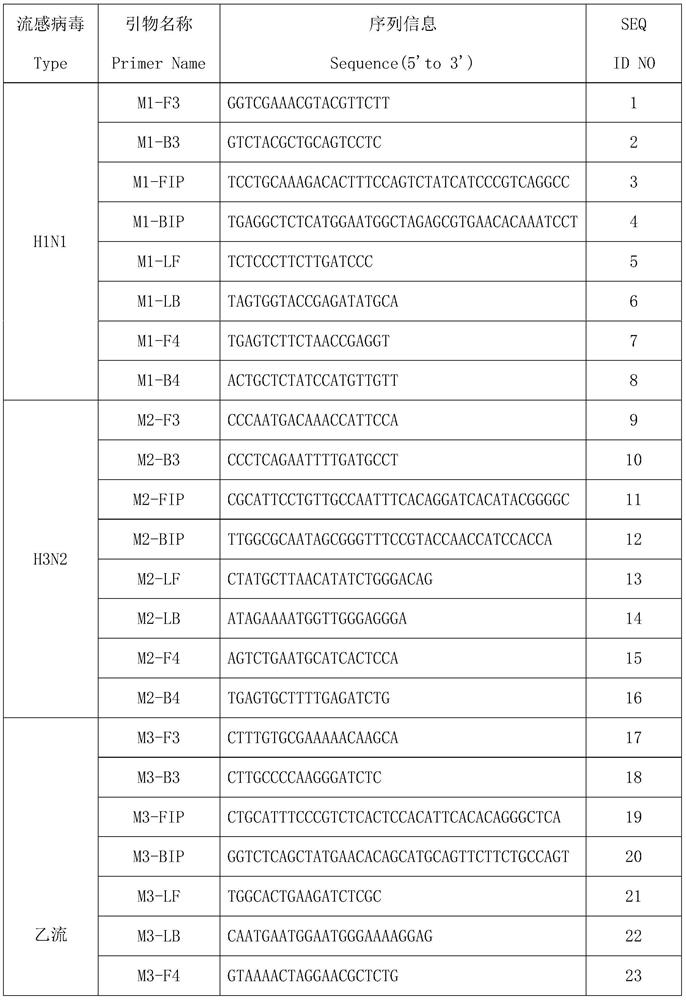
A multiplex lamp primer combination for detecting multiple influenza viruses and its application
ActiveCN113136454BNo false positiveShorten detection timeMicrobiological testing/measurementAgainst vector-borne diseasesMultiplexInfluenza a
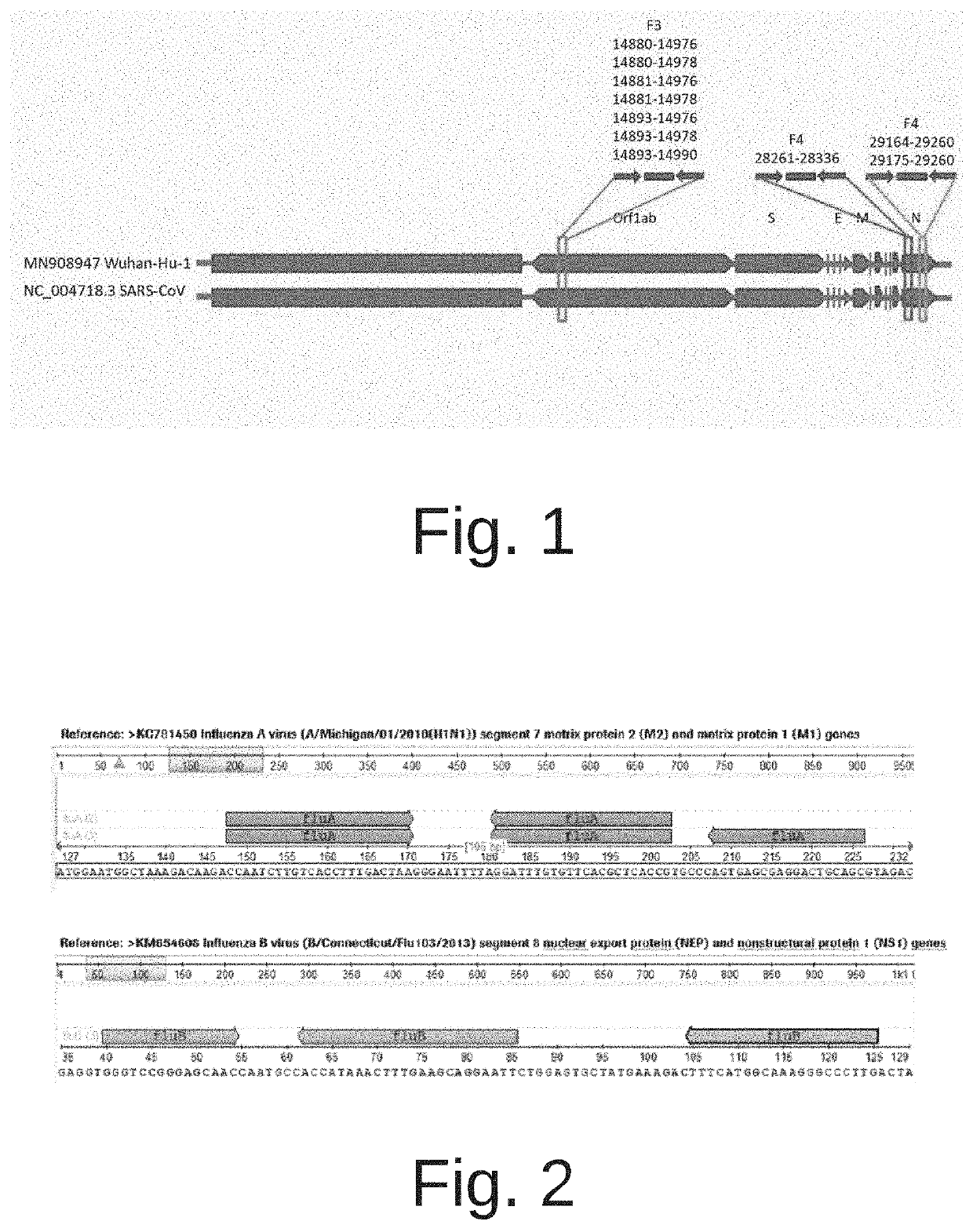
The invention discloses a combination of multiple LAMP primers for detecting multiple influenza viruses and its application. The traditional 2 pairs of primers in 6 regions are improved into 4 pairs of primers in 8 regions, F4 and B4 primers are added, and the detection time is shortened , which improves the sensitivity of detection and does not cause false positives between primers. The multiple LAMP primer combination for detecting multiple influenza viruses provided by the present invention can detect whether it is infected with Influenza A H1N1, Influenza A H3N2 or Influenza B, which is efficient and fast, and is conducive to popularization.
Owner:厦门健康工程与创新研究院
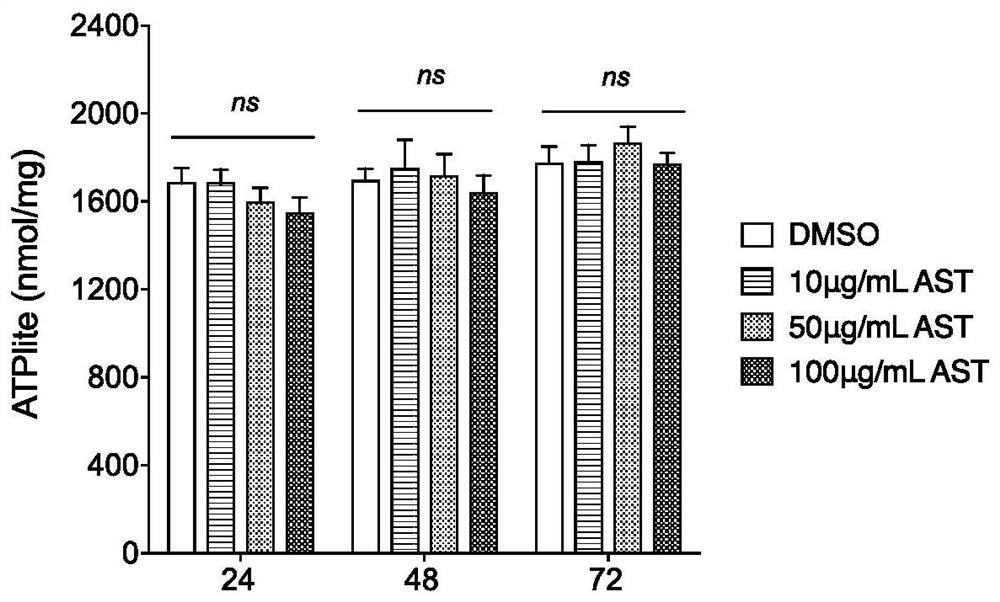
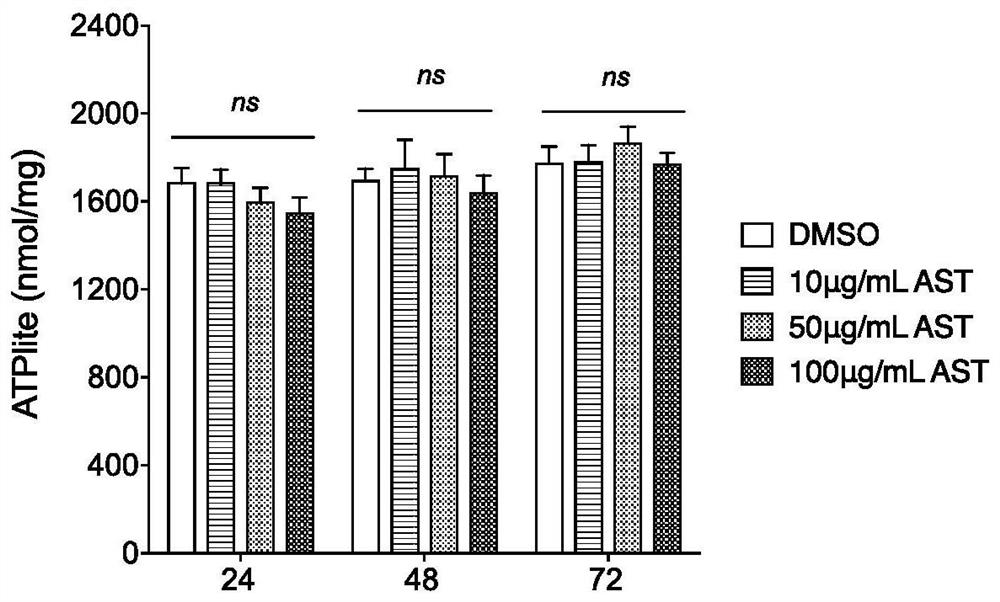
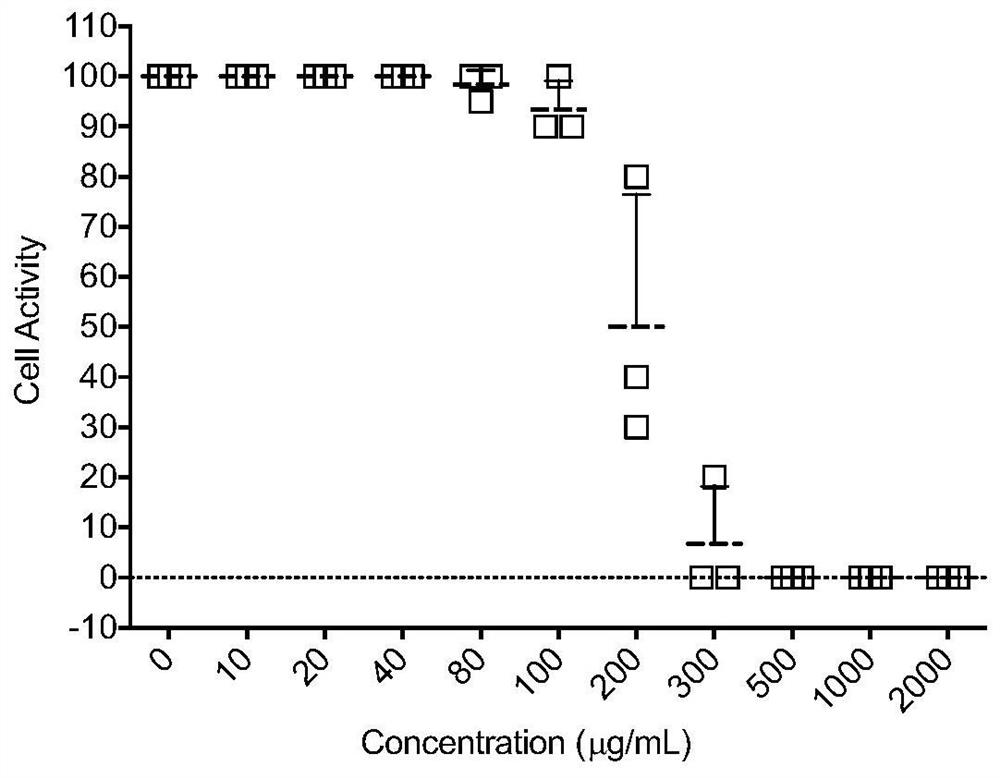
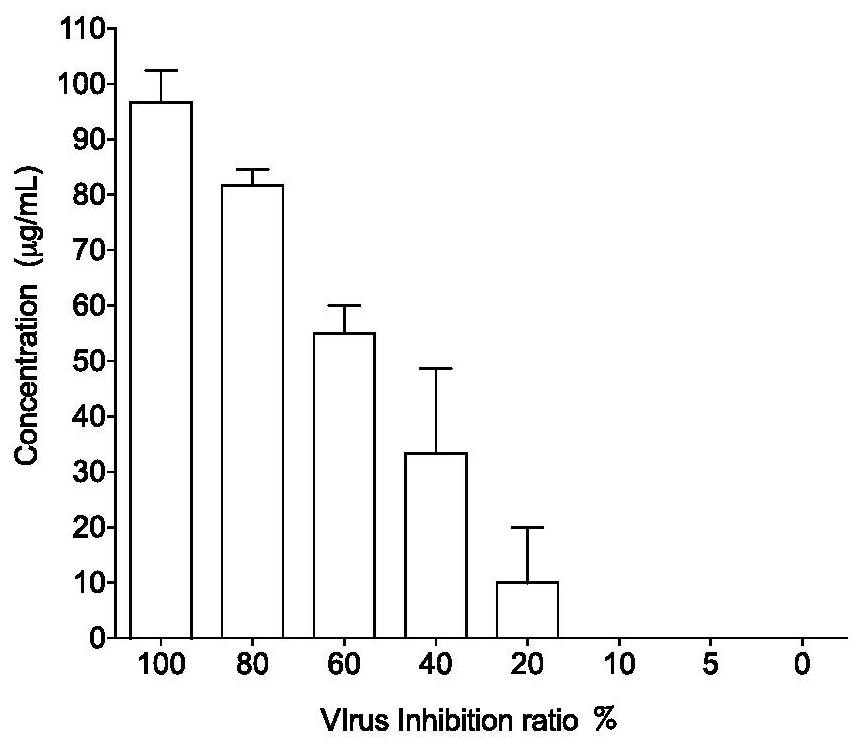
Medicinal use of a kind of astragaloside compound
ActiveCN112353809BEnhanced inhibitory effectGood effectOrganic active ingredientsAntiviralsPharmaceutical SubstancesPharmacology
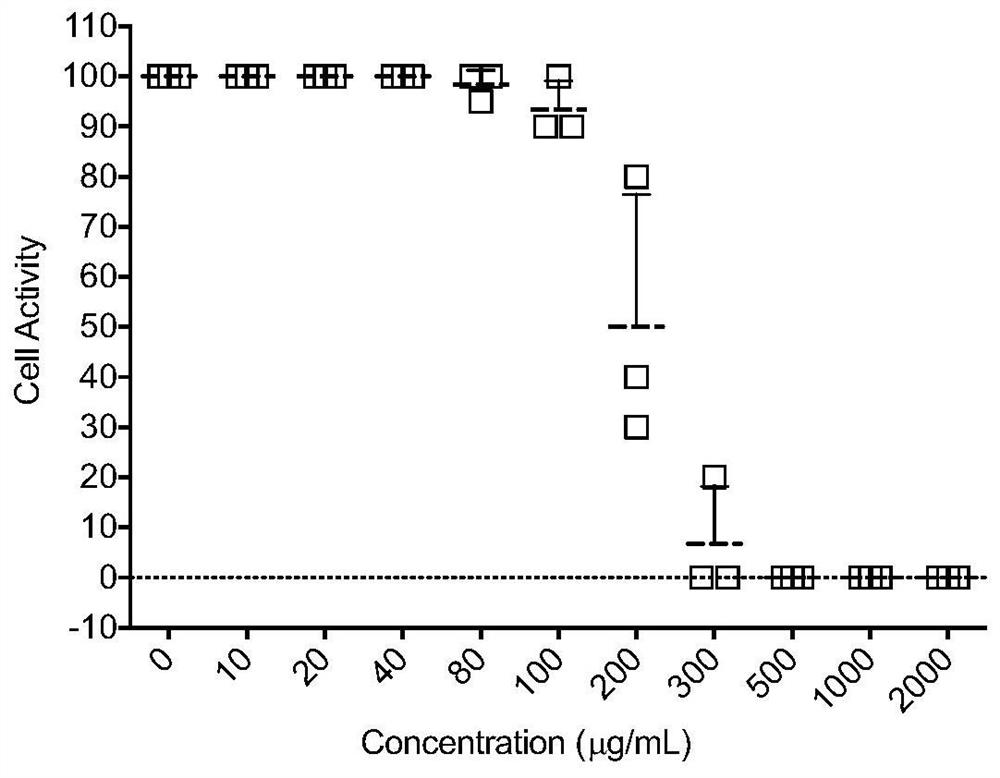
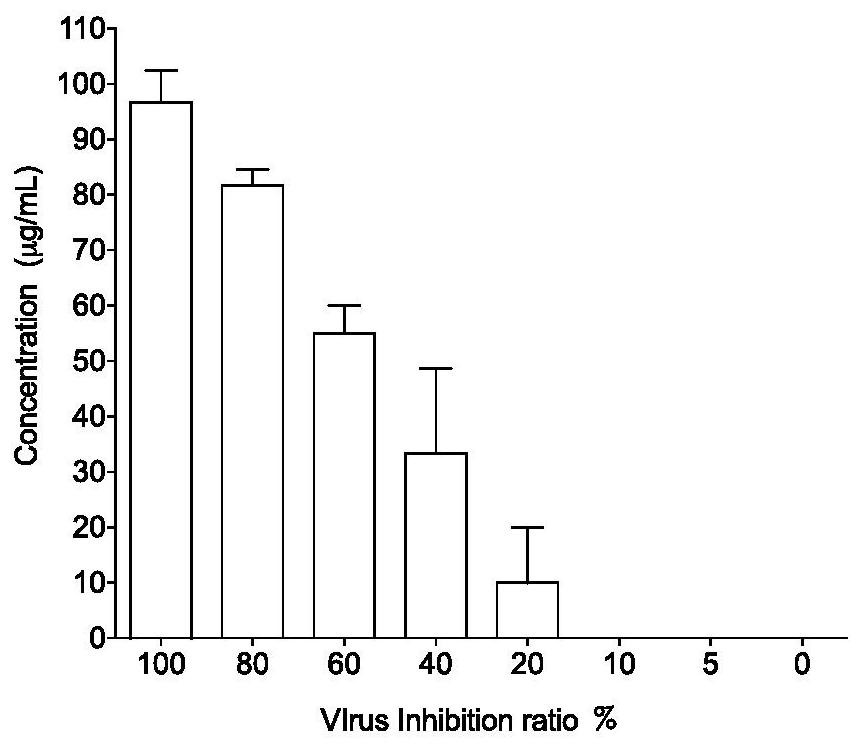
The invention provides an application of an astragaloside IV compound or a pharmaceutical composition containing it in the preparation of anti-influenza B virus medicine. Astragaloside IV AST not only has obvious inhibitory effect on the proliferation of influenza B virus in MDCK cells, but also has no cytotoxic effect within the safe concentration range, and has high safety. It is expected to become a clinical anti-influenza B drug with broad application prospects .
Owner:THE FIFTH PEOPLES HOSPITAL OF SHANGHAI
Human neutralizing antibodies binding to influenza b neuraminidase
The present invention provides influenza neuraminidase (NA)-binding human antibodies, which are capable of specifically binding to and neutralizing at least one influenza B virus strain from the B / Victoria lineage and / or at least one influenza B virus strain from the B / Yamagata lineage, as well as antigen-binding fragment thereof. The invention furthermore relates to the use of said antibodies or antigen-binding fragments in the diagnosis, prophylaxis and / or treatment of influenza infection.
Owner:JANSSEN VACCINES & PREVENTION BV
Probiotic drops for preventing influenza A and B and preparation method thereof
InactiveCN112425781AImprove immunityPromote digestion and absorptionLactobacillusBifidobacteriumBiotechnologySterile environment
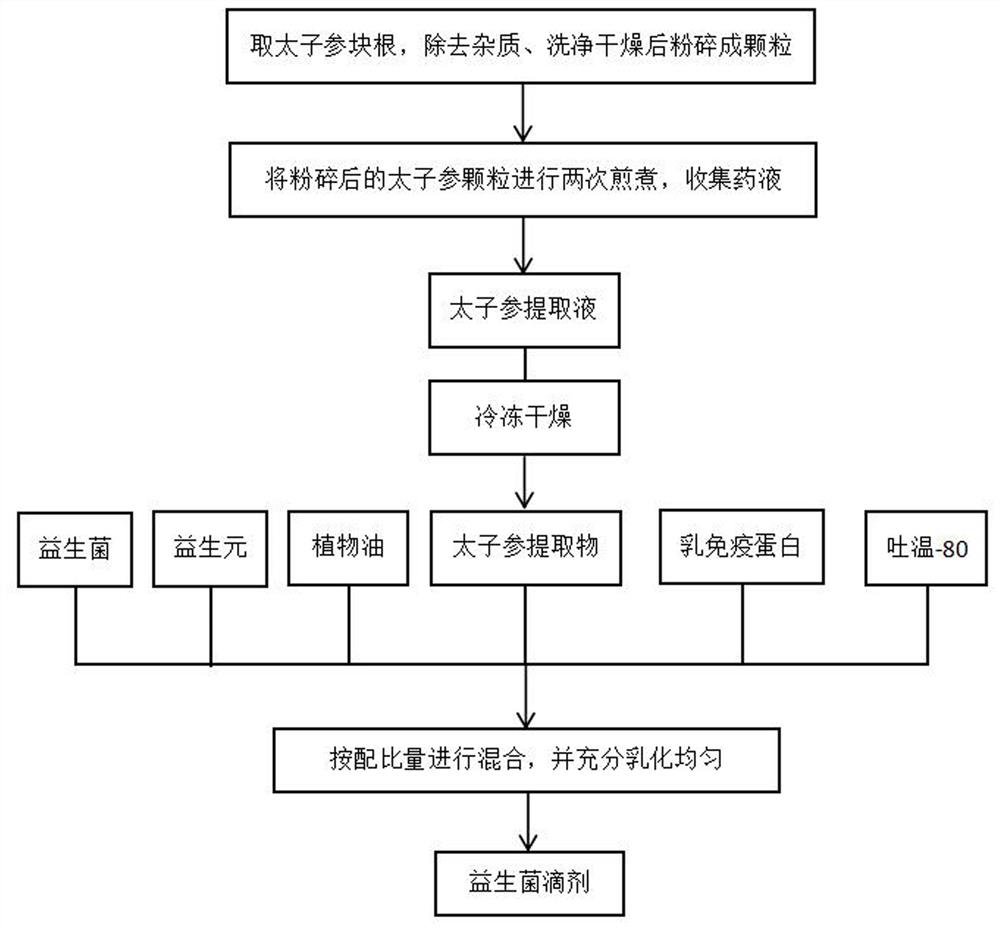
The invention discloses probiotic drops for preventing influenza A and B and a preparation method thereof. The probiotic drops comprise the following components in percentage by mass: 2%-5% of probiotics, 5%-10% of prebiotics, 15% of milk immune protein, 75%-90% of vegetable oil, 15% of a radix pseudostellariae extract and 1%-2% of Tween 80. The preparation method comprises the following steps: firstly preparing the radix pseudostellariae extract, then sterilizing raw materials except the probiotics, finally mixing the probiotics, the prebiotics, the milk immune protein, the vegetable oil, theradix pseudostellariae extract and Tween 80 in proportion in a sterile environment, and performing sufficient and uniform emulsifying to obtain the probiotic drops. The probiotic drops disclosed by the invention contain various beneficial components. On one hand, the probiotic drops can provide nutrient substances capable of enhancing body immunity; and on the other hand, the probiotic drops canimprove intestinal health, regulate and maintain intestinal flora balance, and promote digestive absorption of intestinal tracts on the nutrient substances, so that the immunity of a human body is enhanced, all the components can generate a synergistic effect, and the efficacy of the components can be enhanced.
Owner:RUGBY (GUANGDONG) HEALTH TECH CO LTD +1
One-tube method with multiplex detection for human Influenza A and B and new Influenza A H1N1 virus and kit
ActiveCN101942525BEnable multiple detectionStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationMultiplexInfluenza A antigen
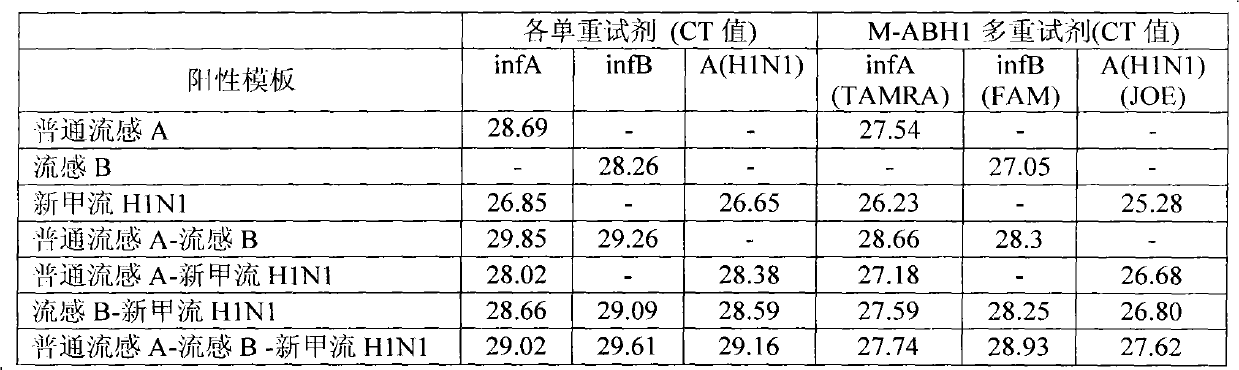
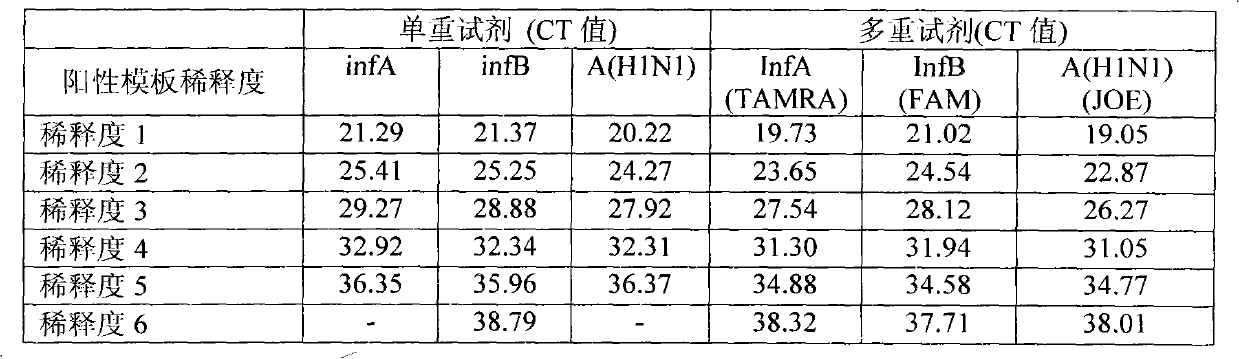
The invention provides a one-tube method with multiplex fluorescent PCR detection for human Influenza A and B and new Influenza A H1N1 virus. The method adopts the primers of which the sequence is shown in SEQ ID NO: 1-6, and also adopts the probes of which the sequence is shown in SEQ ID NO: 7-9. The invention also provides a kit for one-tube method with complex fluorescent PCR detection for human Influenza A and B and new Influenza A H1N1 virus. The kit comprises the primers and the probes. The invention adopts InfA / InfB / A (H1N1) specific primers and Taqman probes, and uses FAM / JOE / TAMRA multiplex fluorescein labels to realize the multiplex detection for the human Influenza A and B and new Influenza A H1N1 virus. The invention has the advantages of high specificity, high sensitivity, high speed, simple and convenient operation, low cost and the like, can be used as a multiple-detection reagent for scientific research and clinical application.
Owner:广东省南山医学发展基金会
Compositions and vaccines against influenza A and influenza B infections
ActiveUS9119810B2SsRNA viruses negative-senseViral antigen ingredientsInfluenza A antigenTGE VACCINE
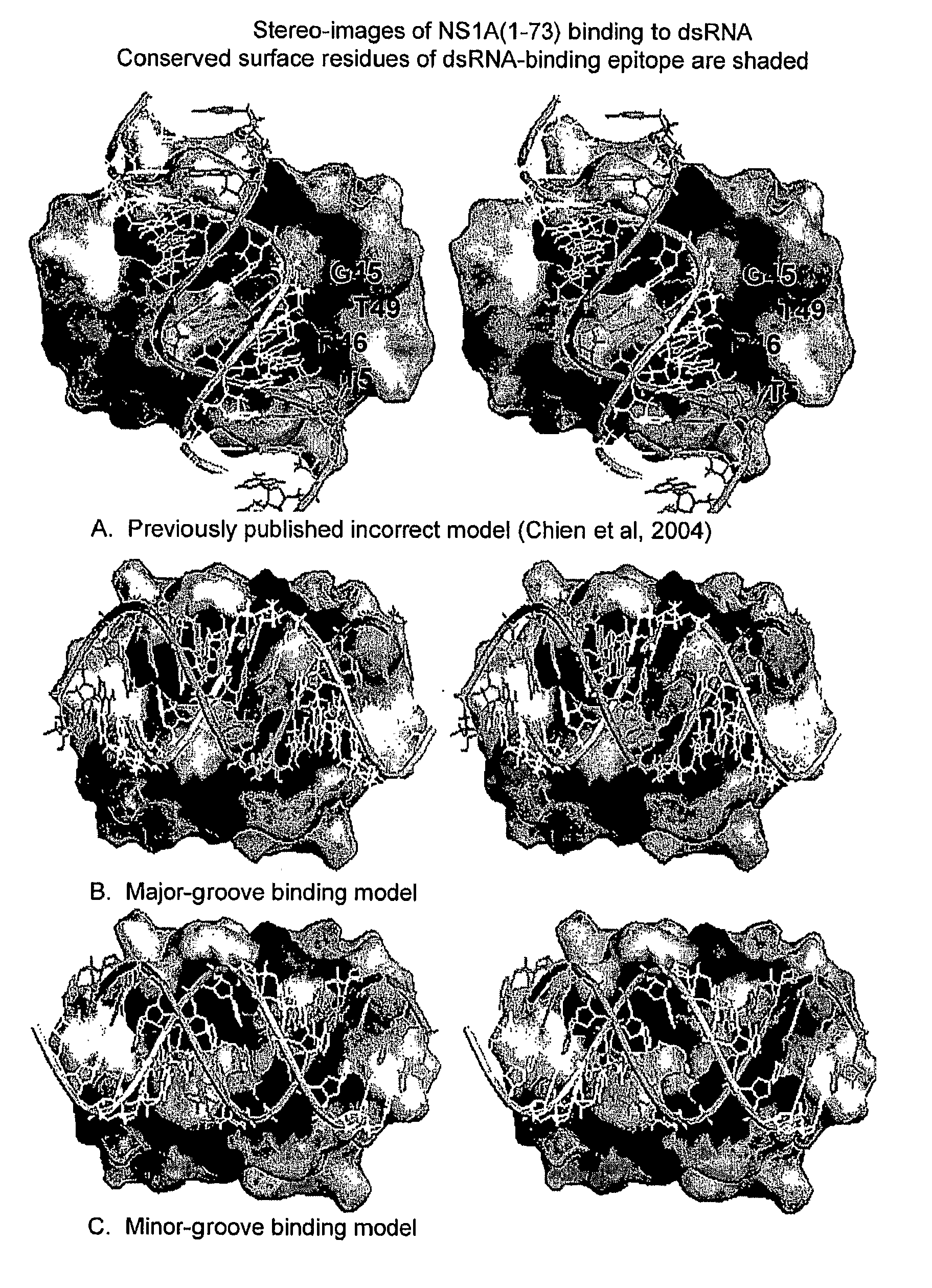
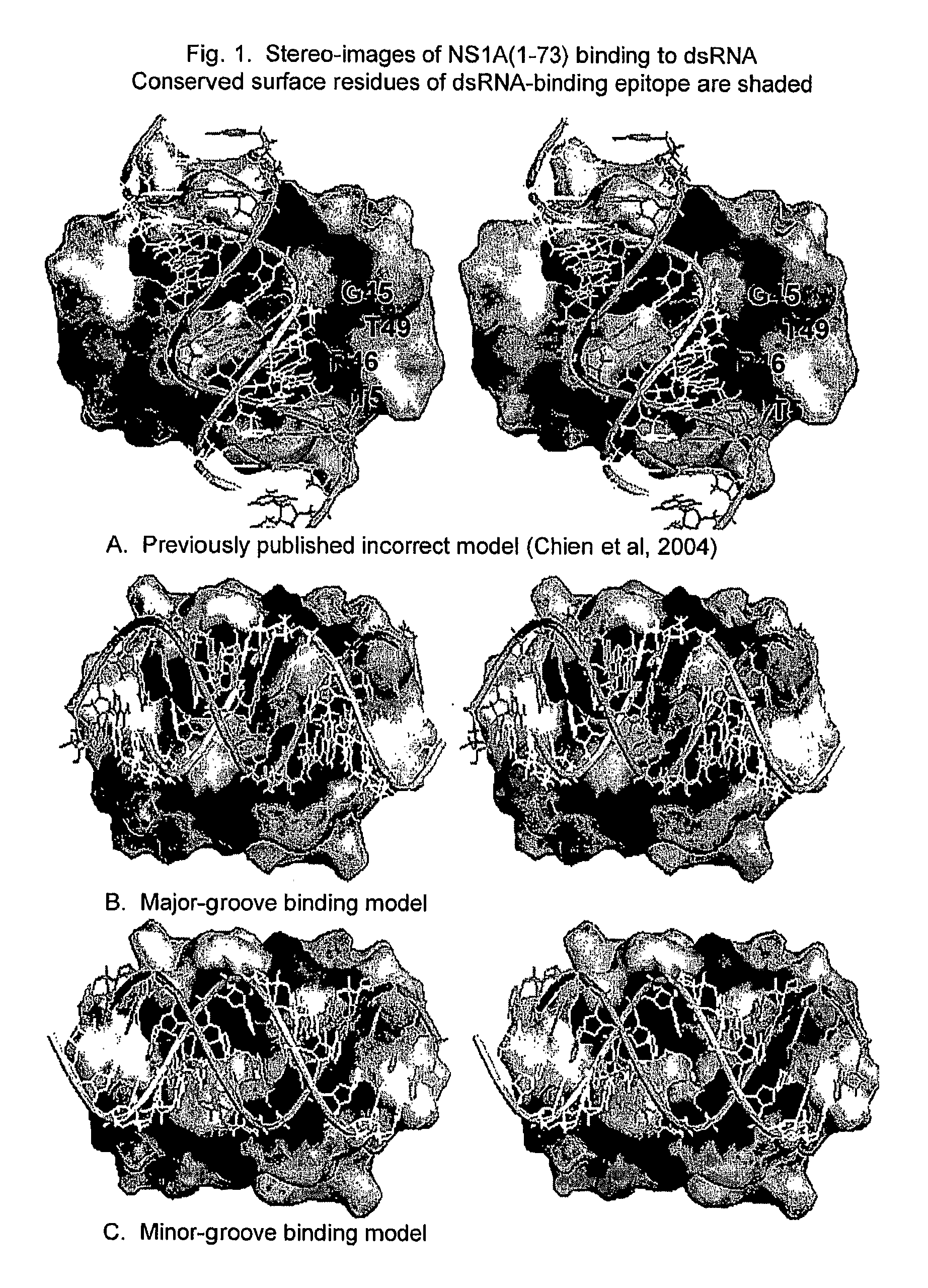
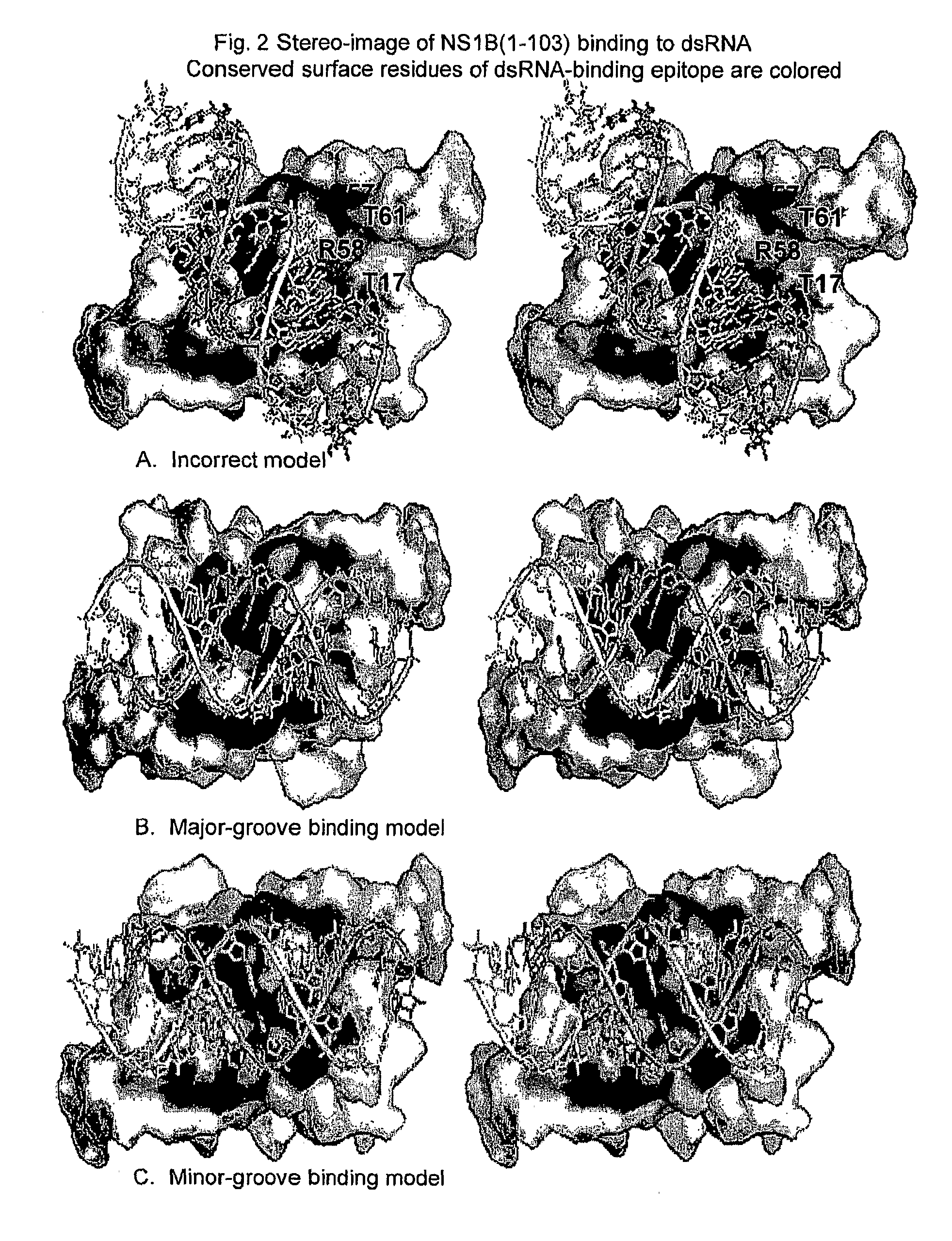
Novel models of interactions of the Nonstructural Protein of influenza A and influenza B viruses (NS1A and NS1B, respectively) with dsRNA are presented. On the basis of the models, novel recombinant viruses and vaccines against influenza A and influenza B viruses are provided.
Owner:RUTGERS THE STATE UNIV
Composition, kit and method for detecting and typing influenza virus and application of composition, kit and method
PendingCN114807434AMicrobiological testing/measurementMicroorganism based processesVirus detectionBioinformatics
The invention belongs to the field of molecular biological detection. In particular, the invention relates to the detection and typing of influenza viruses. More specifically, the invention relates to the detection and typing of influenza A virus. The invention provides a composition for detecting and typing influenza viruses, application of the composition, a kit comprising the composition and a method for using the composition. By using the composition disclosed by the invention, the influenza A / B does not need to be amplified by a plurality of pairs of primers, the influenza A / B can be simultaneously detected by only one pair of primers, the cost is low, the amplification is more uniform, the whole system is more accurate and stable, and meanwhile, the compatibility of the composition disclosed by the invention with other virus detection systems is better.
Owner:SANSURE BIOTECH INC
High-yield recombinant influenza B virus strain and its application
The present invention relates to a kind of new influenza B virus complete gene sequence with mammalian cell high-yield characteristics and carrier containing said equence and strain. Said invention also relates to the influenza B vaccine prepared by using the described strain.
Owner:中国疾病预防控制中心病毒病预防控制所
Pharmaceutical application of astragaloside compound
ActiveCN112353809AEnhanced inhibitory effectGood effectOrganic active ingredientsAntiviralsAstragalosideIntracellular
The invention provides an application of an astragaloside compound or a pharmaceutical composition containing the astragaloside compound in preparation of an anti-influenza B virus drug. AstragalosideAST not only has an obvious inhibiting effect on proliferation of influenza B virus in MDCK cells, but also has no cytotoxic effect in a safe concentration range, is high in safety, is expected to become a clinical anti-influenza B medicine, and has a very wide application prospect.
Owner:THE FIFTH PEOPLES HOSPITAL OF SHANGHAI
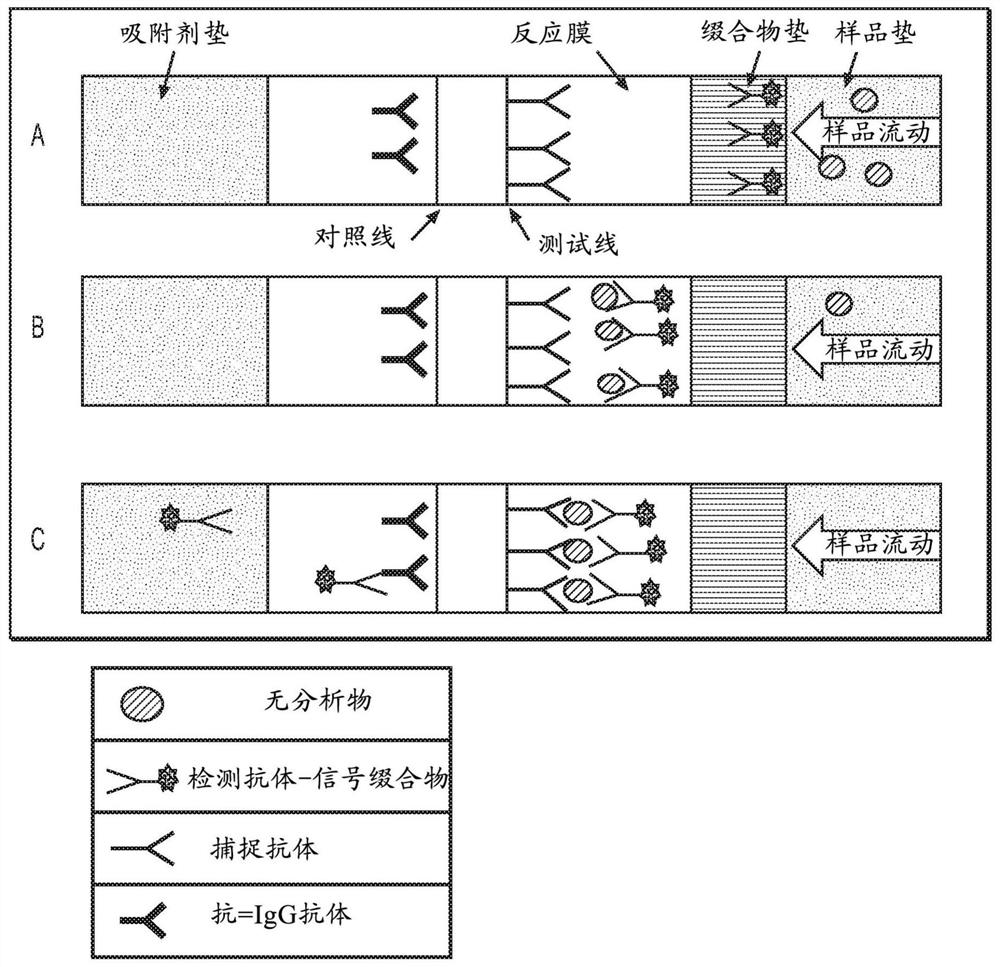
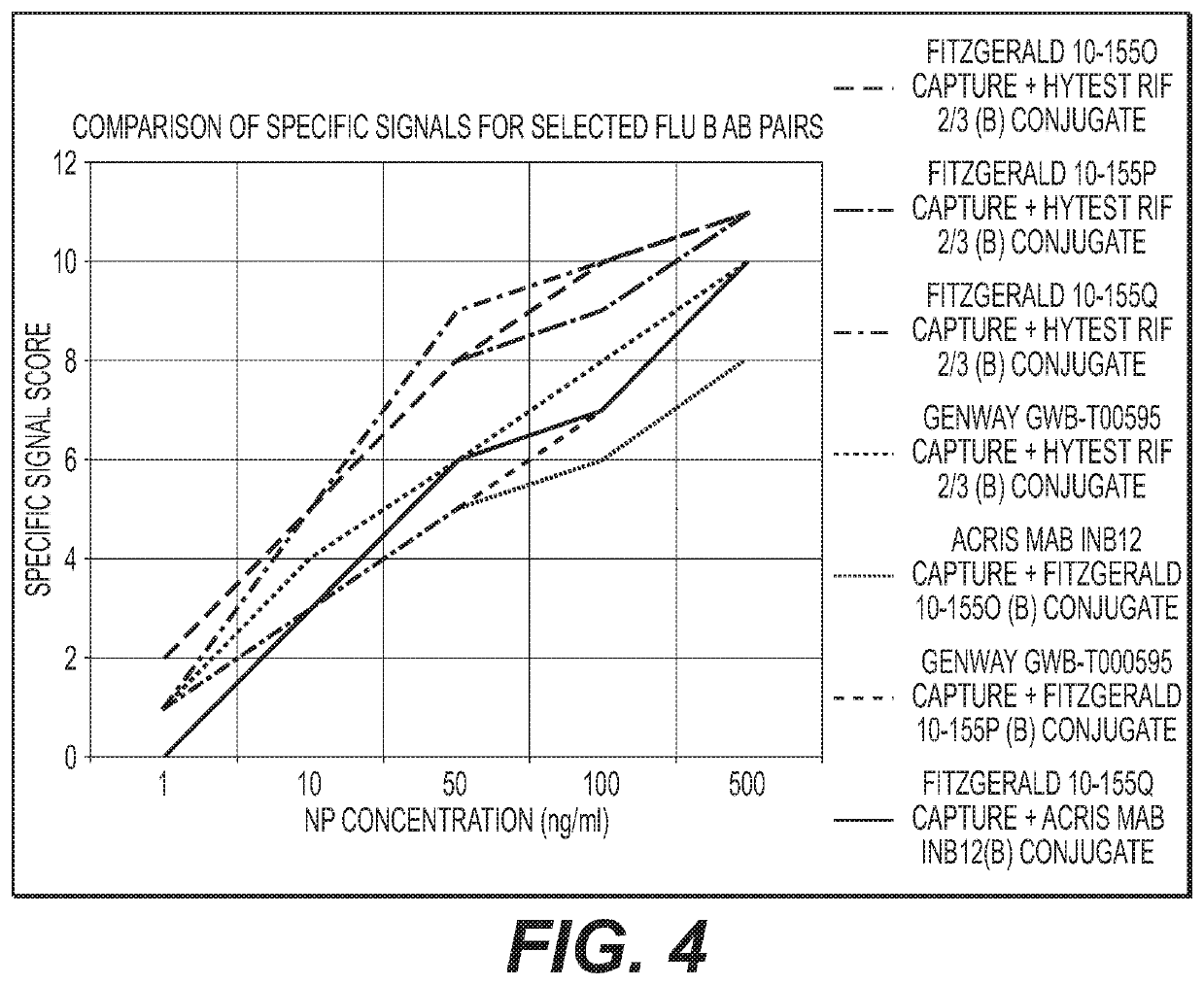
Antibody pairs for use in a rapid influenza b diagnostic test
Owner:GLAXOSMITHKLINE CONSUMER HEALTHCARE HLDG US
Methods of making and using universal centralized influenza vaccine genes
PendingCN113423421ASsRNA viruses negative-senseSsRNA viruses positive-senseInfluenza A antigenNucleic acid sequencing
This disclosure describes a number of different polypeptide sequences, and the nucleic acid sequences encoding such polypeptide sequences, that can be used alone or in combination as universal vaccines (e.g., against influenza A or influenza B in humans or influenza in swine).
Owner:BOARD OF RGT UNIV OF NEBRASKA
Antibody pairs for use in a rapid influenza b diagnostic test
PendingUS20210247395A1Prevent backflowImprove stabilityDisease diagnosisImmunoglobulinsDiagnostic testBiomedical engineering
Owner:GLAXOSMITHKLINE CONSUMER HEALTHCARE HLDG US
Multiple LAMP (loop-mediated isothermal amplification) primer combination for detecting multiple influenza viruses and application of multiple LAMP primer combination
ActiveCN113136454ANo false positiveShorten detection timeMicrobiological testing/measurementAgainst vector-borne diseasesBioinformaticsInfluenza a
The invention discloses a multiple LAMP (loop-mediated isothermal amplification) primer combination for detecting multiple influenza viruses and application of the multiple LAMP primer combination, the traditional 6-region 2-pair primers are improved into 8-region 4-pair primers, and F4 and B4 primers are added, so that the detection time is shortened, the detection sensitivity is improved, and false positive cannot be caused among the primers. The multiple LAMP primer combination for detecting multiple influenza viruses provided by the invention can be used for detecting whether influenza A H1N1, influenza A H3N2 or influenza B is infected, is efficient and rapid, and is beneficial to popularization.
Owner:厦门健康工程与创新研究院
Buffer solution for cas12a editing DNA as well as preparation method and application of buffer solution
PendingCN113604608ACause degradation, etc.High sensitivityMicrobiological testing/measurementDNA/RNA fragmentationGlycerolCombinatorial chemistry
The invention discloses a buffer solution for cas12a editing DNA as well as a preparation method and application of the buffer solution. The buffer solution is prepared from the following raw materials: 5 to 25 mM of HEPES with the pH of 6.8 to 7.7, 80 to 300 mM of KCl, 5 to 20 mM of MgCl2, 0.5 to 2 percent of glycerol and 0.3 to 0.7 mM of DTT. The preparation method comprises the following steps: preparing an HEPES solution with the required concentration, adjusting the pH value, sequentially adding KCl, MgCl2, glycerol and DTT according to the required concentration, and uniformly mixing to obtain the buffer solution. The HEPES in the invention belongs to a high-purity biological buffering agent, and does not cause degradation and other influences on all reaction components; the buffer solution provided by the invention can increase the sensitivity of cas12a for detecting influenza B DNA by 10-100 times, and the highest sensitivity can reach 100 fM; and the preparation technology is simple, operation is convenient, and high practicability is achieved.
Owner:天益健康科学研究院(镇江)有限公司
Anti-hemagglutinin antibodies and methods of use thereof
Provided herein are monoclonal antibodies, or antigen-binding fragments thereof, that bind to the influenza B hemagglutinin (HA) protein, pharmaceutical compositions comprising the antibodies, and methods of use. The antibodies can be used to inhibit or neutralize the activity of influenza B virus, thereby providing a method of treating or preventing influenza infection in humans. The use of one or more antibodies that bind to the influenza B HA for preventing viral adsorption and / or entry into host cells is also provided. The antibodies can be used prophylactically or therapeutically and can be used alone or in combination with one or more other antiviral agents or vaccines.
Owner:REGENERON PHARM INC
Live attenuated influenza b virus compositions methods of making and using thereof
PendingUS20220016232A1SsRNA viruses negative-senseViral antigen ingredientsVaccine ProductionLive attenuated influenza vaccine
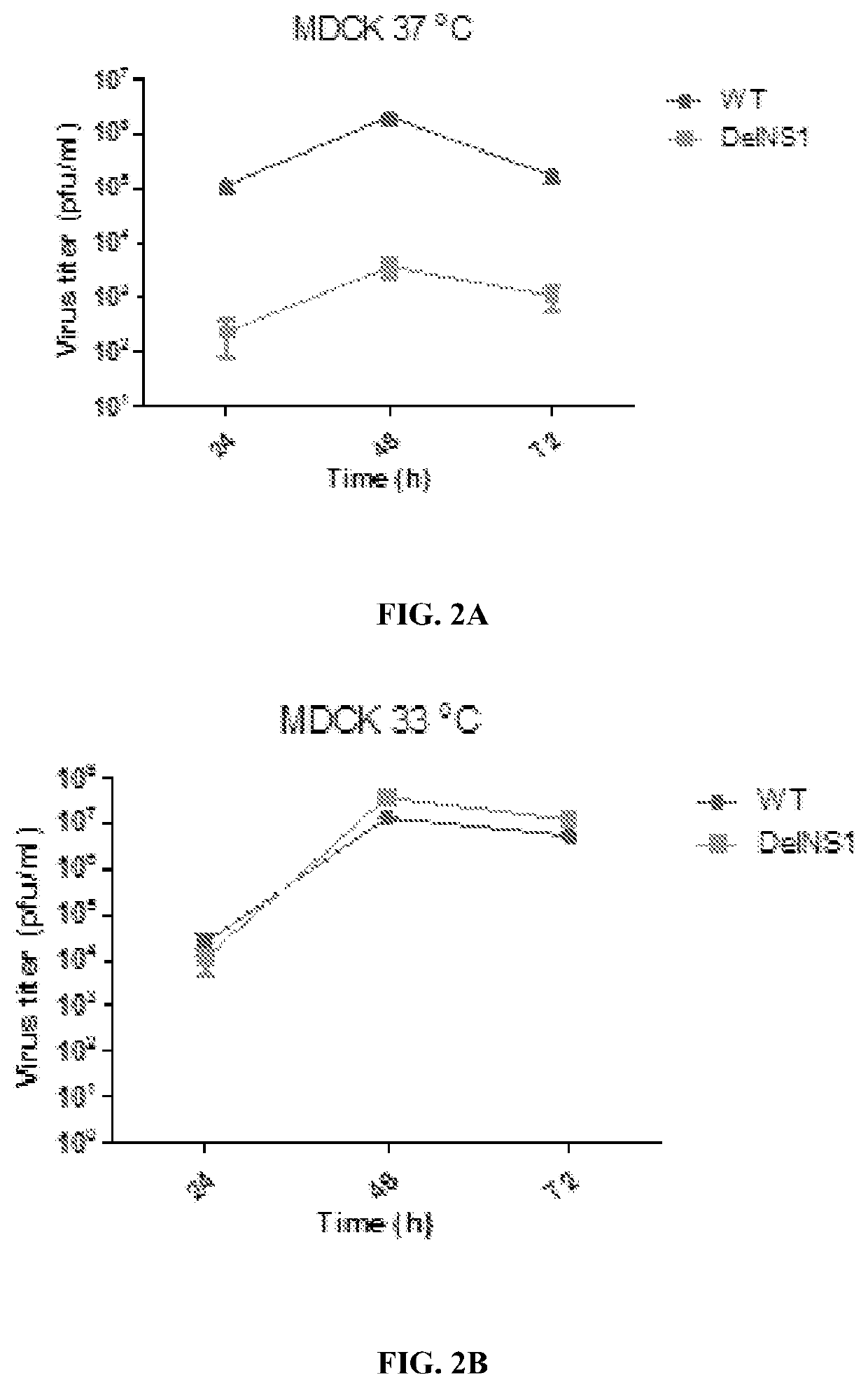
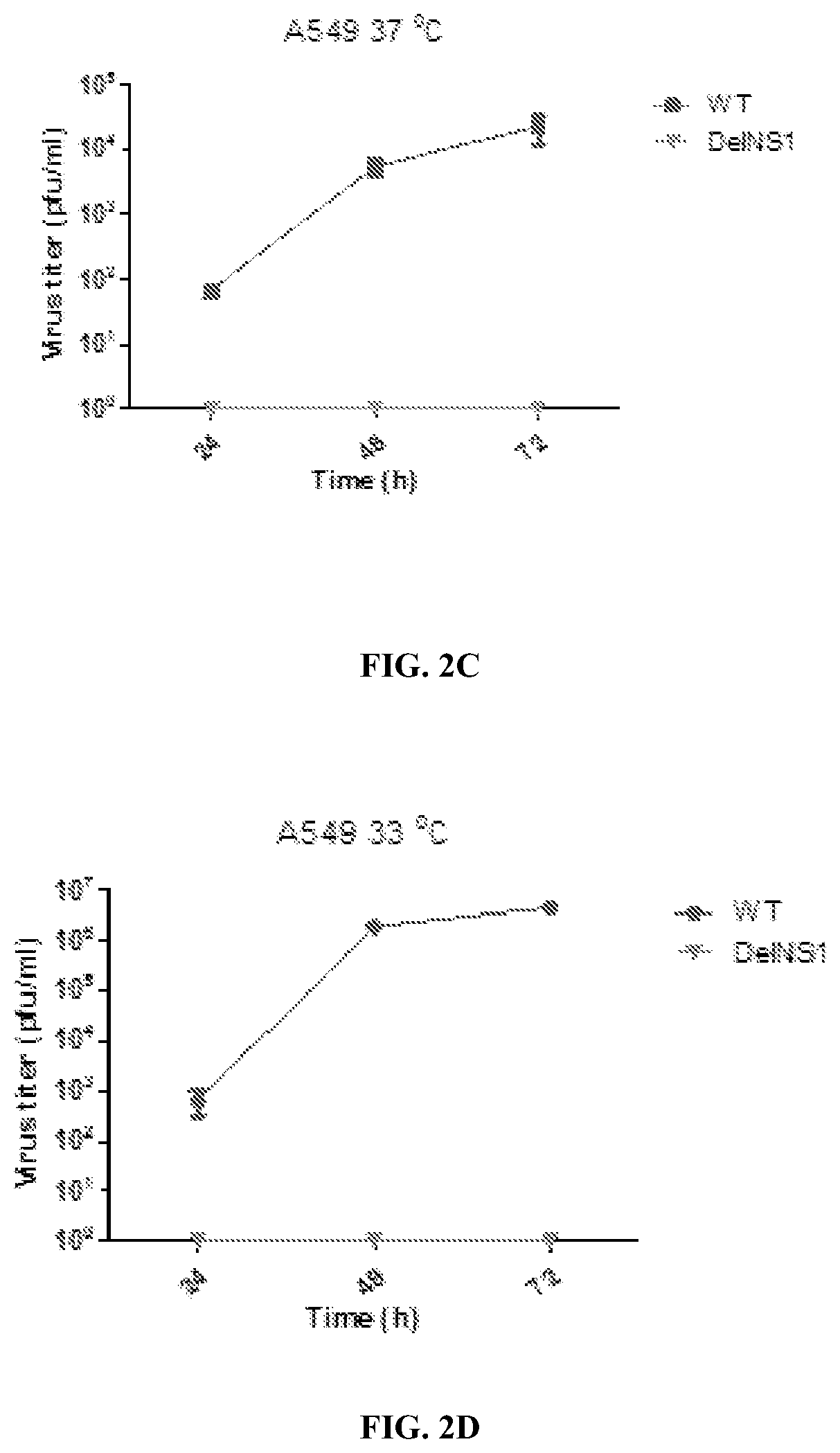
The NS1 protein of influenza virus is a key virulent element with multi-functional roles in virus replication and acts as a strong interferon (IFN) antagonist. A live attenuated virus (LAIV) is provided using a master backbone, which contains the influenza B (HK8038) virus and includes a deletion of the NSI coding region (DelNS1). The LAIV is based on novel adaptive mutations, which support DelNS1 influenza B live attenuated virus (LAIV) replication in vaccine producing cells. DelNS1 influenza B LAIV shows spontaneous cold adaption with preference to grow at 30-33° C. but restriction at 37-39° C. The LAIV can be used to protect a subject, against a lethal challenge of antigenic distant influenza B viruses. DelNS1 LAIV with adaptive mutations for growing in vaccine producing systems is an important strategy for making highly attenuated and immunogenic live attenuated influenza vaccines with the ability to induce broad cross protective immunity for seasonal influenza.
Owner:VERSITECH LTD
Epidemic prevention traditional Chinese medicine composition and sachet prepared from same
InactiveCN112472744AReasonable formulaInhibition of activityPowder deliveryHydroxy compound active ingredientsAgastache rugosaAtractylodes chinensis
The invention relates to the technical field of traditional Chinese medicines, in particular to an epidemic prevention traditional Chinese medicine composition and a sachet prepared from the same. Theepidemic prevention traditional Chinese medicine composition is prepared from fructus forsythiae, cortex phellodendri, flos lonicerae, herba taraxaci, centipede, folium artemisiae argyi, agastache rugosus, herba eupatorii, radix angelicae dahuricae, radix aucklandiae, rhizoma atractylodis, herba menthae and menthol. The sachet prepared from the epidemic prevention traditional Chinese medicine composition can achieve the remarkable effect of preventing common cold, influenza A and influenza B.
Owner:SHANDONG HANFANG PHARMA
Method for measuring influenza B virus
ActiveUS11360088B2Easy to detectImmunoglobulins against virusesImmunoassaysAntigenSpecific detection
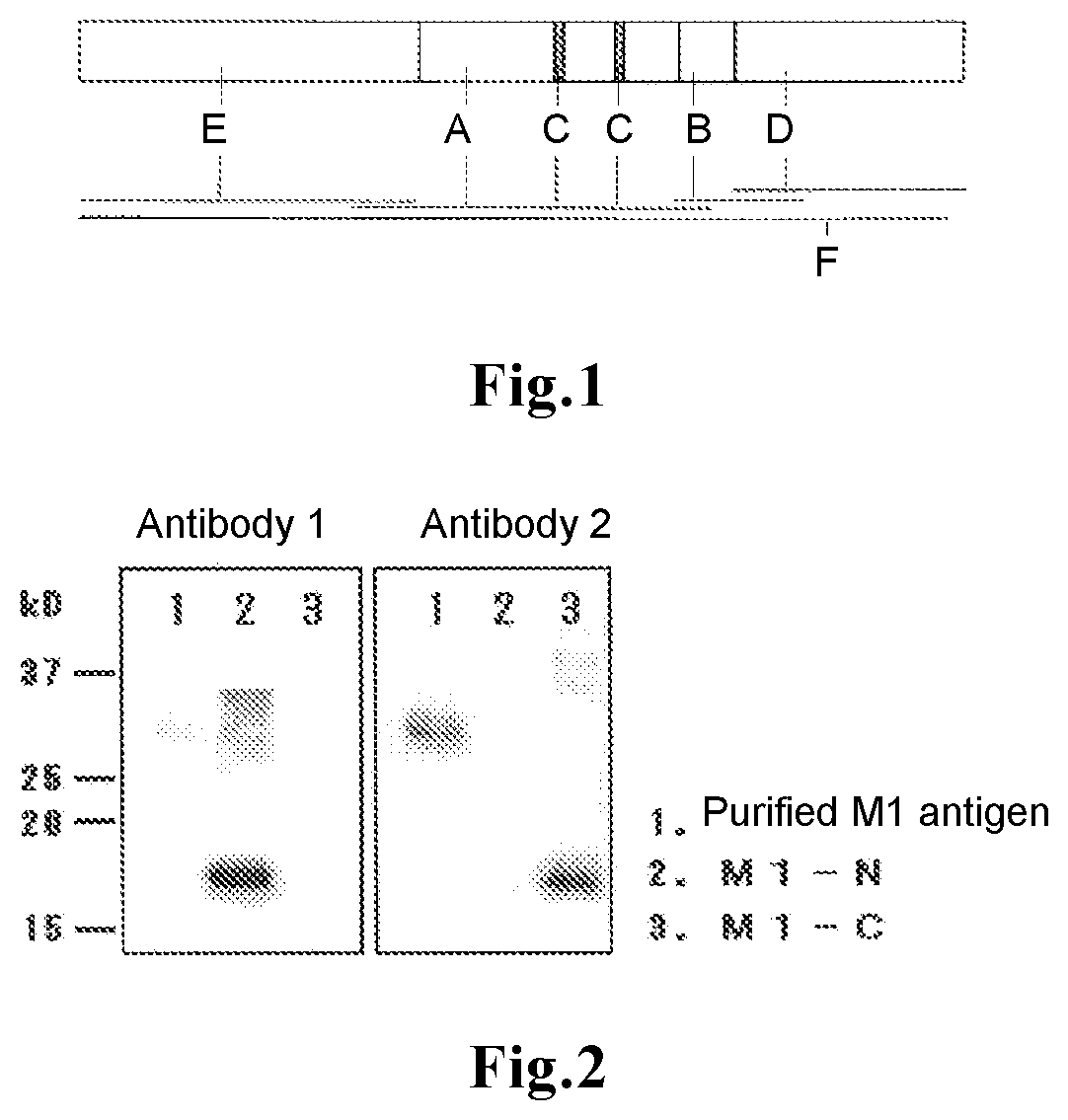
A method for measuring influenza B virus by an immunoassay, which method enables specific detection of influenza B virus with a higher sensitivity than conventional methods, and a device or a kit therefor are disclosed. The method for measuring influenza B virus includes carrying out an immunoassay of influenza B virus by a sandwich method using two kinds of monoclonal antibodies each of which specifically reacts with the region of the 125th to 248th amino acids of matrix protein (M1) of influenza B virus, which two kinds of monoclonal antibodies are capable of binding to the region of the 125th to 248th amino acids of M1 at the same time, or antigen-binding fragments thereof.
Owner:DENKA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com