One-tube method with multiplex detection for human Influenza A and B and new Influenza A H1N1 virus and kit
A type of influenza B, influenza virus technology, applied in biochemical equipment and methods, recombinant DNA technology, microbial determination/inspection, etc. High specificity, low cost, simple and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the specific experiment of primer, probe
[0045]Using bioinformatics and biological software for designing primers and probes, we designed primers and probes for the conserved regions of influenza A and B viruses on the sequence data of influenza A, B and new influenza A H1N1 viruses, and designed primers and probes for the new influenza A and H1N1 viruses. Primer probes were designed for the conserved region of the H1 gene of the H1N1 influenza virus, and the probes were labeled with TAMRA, FAM, and JOE respectively. The detailed sequences are shown in Table 1, and Table 1 shows the sequences of primers and probes for human influenza A, influenza B and new influenza A H1N1 viruses.
[0046] Table 1
[0047]
[0048] In order to verify the specificity of the designed primers and probes, a single-plex fluorescent PCR reaction was designed first, and the primers and probes were evaluated, that is, the three sets of primers and corresponding probes of In...
Embodiment 2
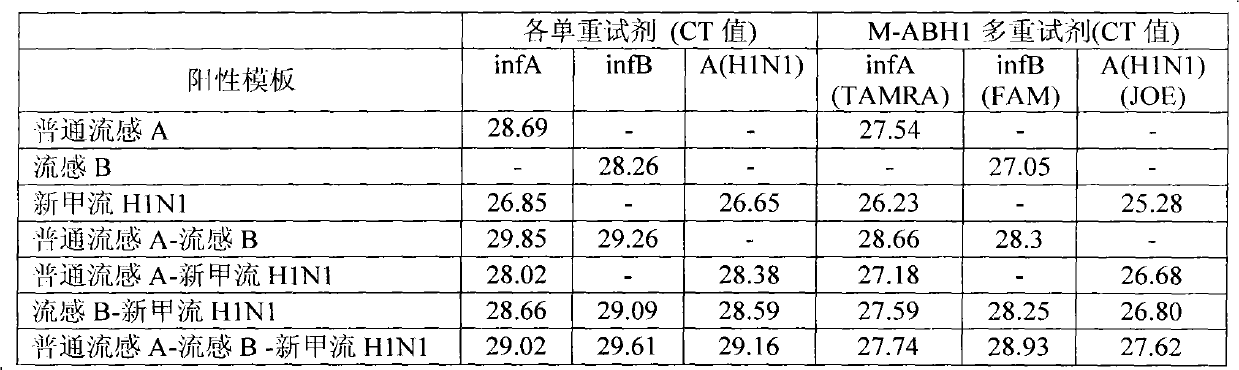
[0052] Embodiment 2: Specificity experiment of M-ABH1 multiple detection reagent
[0053] Multiple detection reagents are prepared using the RT-PCR buffer of TAKARA'PrimeScript One-Step RT-PCR Kit (Ver.2)' (TAKARA'PrimeScriptOneStepRT-PCRKitVer.2'), in which the amount of primer used is 7-15pmol, The amount of probe used is 0.5-5 pmol. Prepare the InfA / InfB / A(H1N1) primers and each probe in Table 1 into a tube of reaction solution (M-ABH1). The total reaction volume is 25 μl / reaction, the volume of prepared reagents is 18 μl / reaction, 2 μl is reserved for adding enzyme mixture; 5 μl is reserved for adding template. Use single positive template (common influenza A; influenza B or new H1N1); double positive template (common influenza A-influenza B; common influenza A-new H1N1; influenza B-new H1N1); triple positive The template (common influenza A-influenza B-new influenza A H1N1) was tested for multiple detection reagents, and single-plex fluorescent PCR reagents were used fo...
Embodiment 3
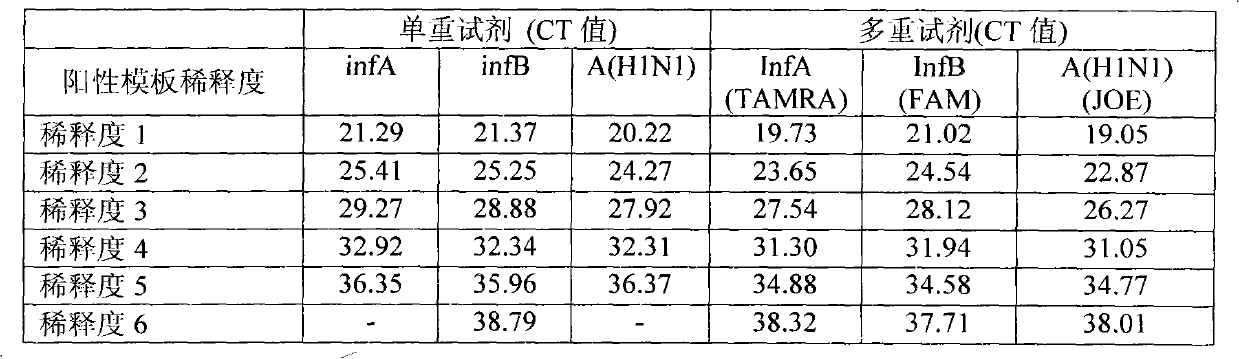
[0057] Embodiment 3: Sensitivity experiment of M-ABH1 multiple detection reagent
[0058] In order to further test the sensitivity of the reagents, a 10-fold gradient dilution was performed on human influenza A (H3N2 type), influenza B and new influenza A H1N1 influenza virus positive samples, which were recorded as dilutions 1 to 6, respectively representing infA-H3N2 , infB, and A(H1N1) viruses were diluted 10 to 106 times, respectively, compared with the single-fold detection with multiple influenza virus detection reagents, and the virus culture detection was carried out at the same time. The experimental results of quantitative PCR are shown in Table 4. The identifiable range of virus culture is at dilution 4. When the dilution is 5, the culture method is negative, and the sensitivity is far lower than that of single or multiplex influenza quantitative PCR detection. Table 4 is a data table of comparative experimental results of multiplex and singlex parainfluenza quanti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com