Methods of making and using universal centralized influenza vaccine genes
A vaccine and vaccination technology, applied in the field of vaccines, can solve serious pneumonia, death and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0228] Example 1 - Viral gene and generated vaccine sequence
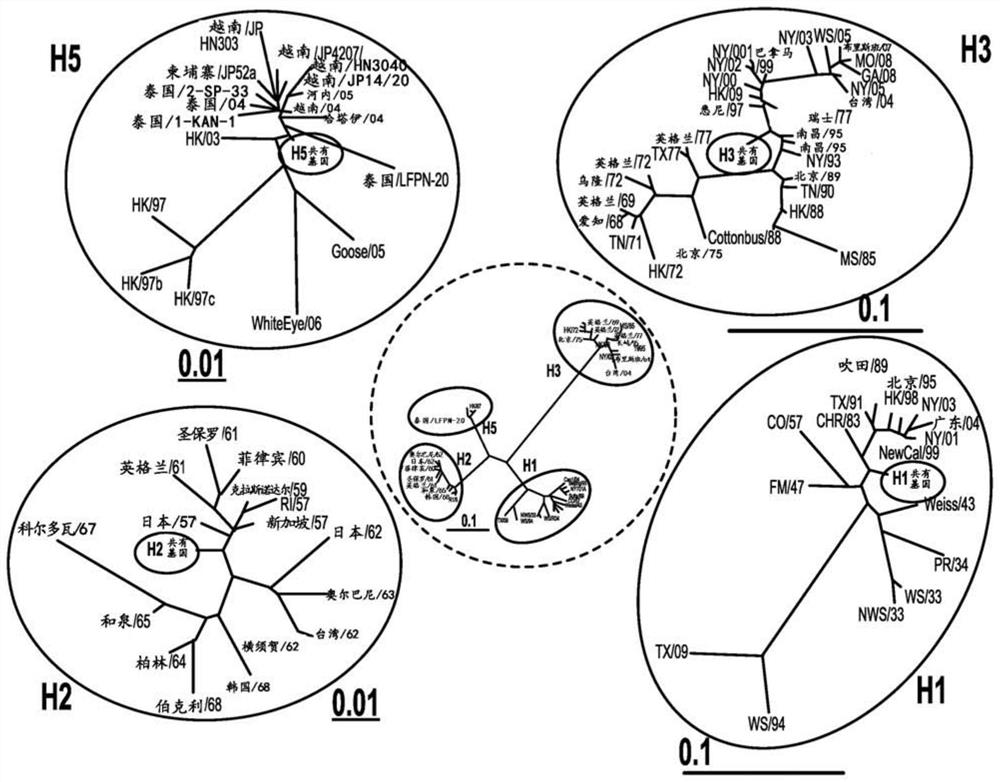
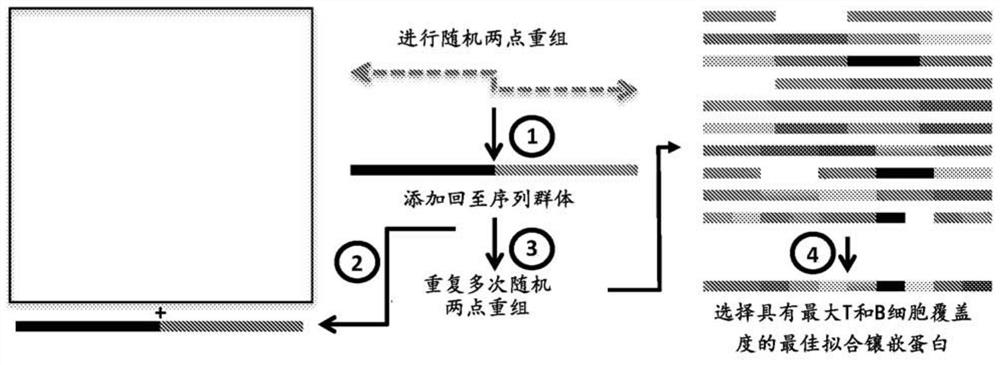
[0229] The genetic relationship between the vaccine gene and the wild-type circulating gene described herein is shown in Figures 9- 23. Since the vaccine genes described herein are prepared using all unique HA sequences, they are unlike any genes prepared using other strategies such as common sequence, Cobra, Mosaics, and COT.
[0230] The unique universal vaccine gene described herein is used to resist human A influenza viruses (H1, H2, H3, H5, N1 and N2 (SEQ ID NO: 1-43)), human fluorinated virus (HA mountain shape, HA Victoria, Na Mountain Class, NA Victoria (SEQ ID NO: 44-74)) and pig Aflu virus (H1, H3, N1 and N2 (SEQ ID NO: 75-102)), and can be applied to A general influenza vaccine is produced for all known influenza strains. Nucleic acid sequences encoding representative flu vaccines are shown in SEQ ID NO: 103-122.
Embodiment 2
[0231] Example 2 - Preliminary results
[0232] The Animal Use protocol approved by the Association for Assessment Acreditation of Laboratory Animal Care (Aalac) is used according to the Laborative Animal Care, Aalac. The ASSOCIMAL CARE, AALAC is used to place all animals in UNL Life Sciences Annex. All animal experiments are in accordance with the provisions of Animal Welfare Act, PHS Animal WelfarePolicy, NiH Guide for the Care and Use Oflaborative Animals, and UNL Policy Cuts with procedures.
[0233] Gender as a biological variable. Only female mice were used in vivo studies. However, all successful vaccine candidates were confirmed in the study of male mice.
[0234] Biological hazard. All biohazard materials have been approved by the UNL mechanism Biosafety Committee (IBC). UNL IBC is responsible for the safe use of the infection and recombinant DNA in the UNL laboratory. This study did not use any reagents that rating higher than BSL2.
[0235] Strictness and transparency....
Embodiment 3
[0269] Example 3 - Representative sequence comparison
[0270] As shown in Table 1, sequences of the selected vaccine polypeptides described herein are compared to each other.
[0271] Table 1. Comparison of the sequence of influenza H1 HA protein sequence
[0272]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com