Pharmaceutical application of astragaloside compound
A technology of astragaloside IV and medicine, applied in the field of medicine, can solve the problems such as difficulty in preventing and controlling influenza
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
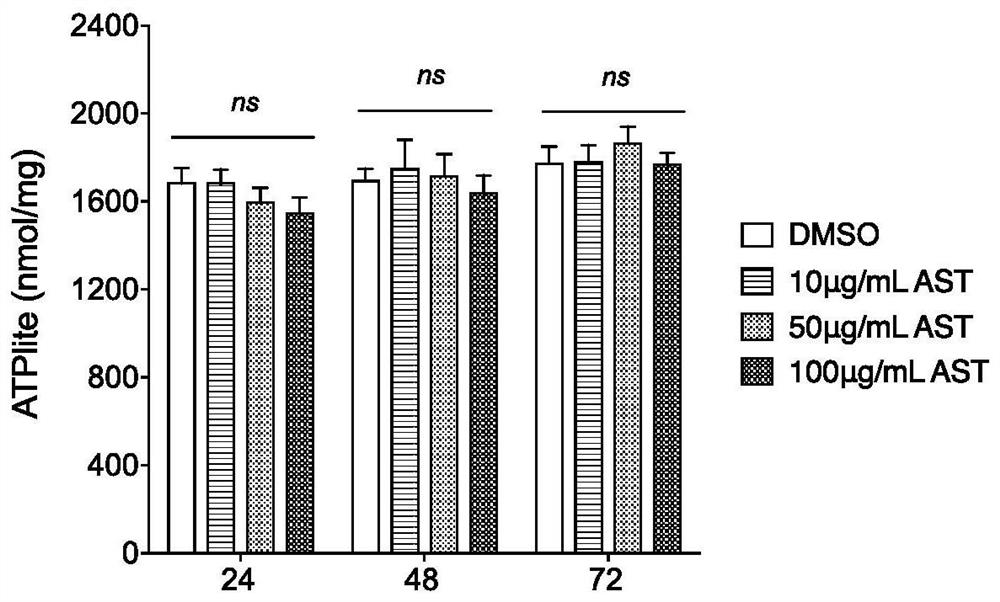
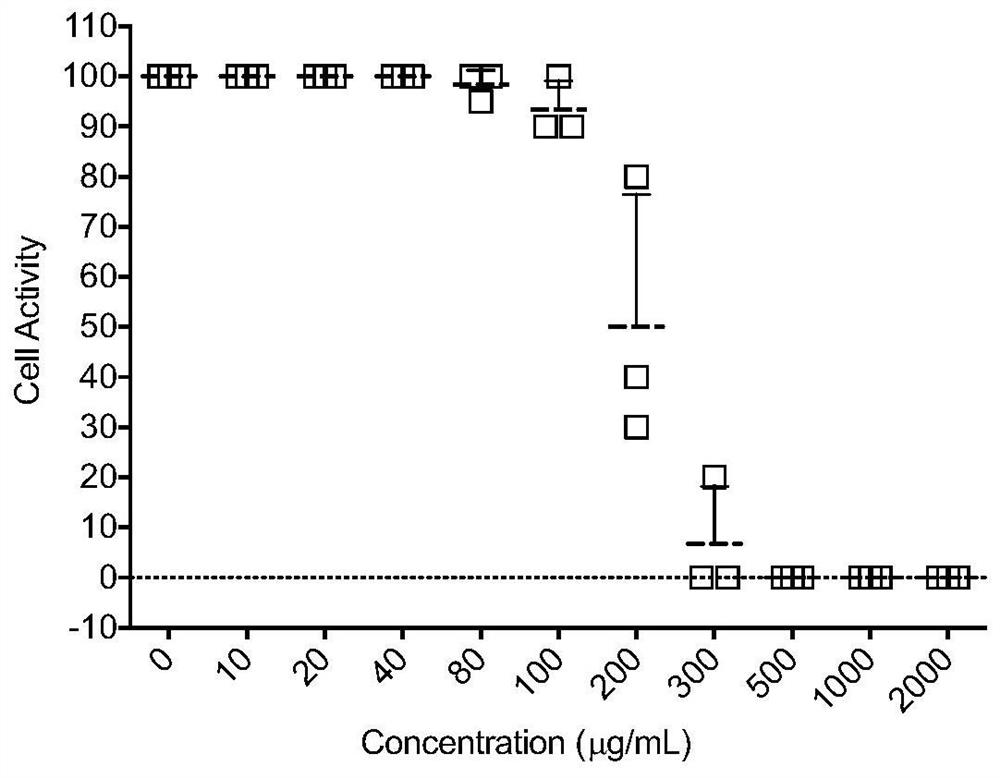
[0030] Embodiment 1 Astragaloside IV (AST) is to cytotoxicity assay experiment
[0031] When astragaloside IV AST was applied to MDCK cells at concentrations of 5 μg / ml, 10 μg / ml, 20 μg / ml, 40 μg / ml, 60 μg / ml, 80 μg / ml, and 100 μg / ml for 72 hours, the MDCK cells were treated at a concentration of astragaloside IV AST of 80 μg. When the concentration is 100μg / ml, there is some rounding under the light microscope, but there is no obvious toxicity, and when the concentration is 100μg / ml, there is obvious cell rounding. When the concentration is higher than 100μg / ml, the cytotoxicity is more obvious, and there is obvious cell aggregation phenomenon. The OD value of each hole was measured by MTT method, and the results showed that: when the concentration of MDCK cells was 100 μg / ml, the survival rate was more than 93%, while at 200 μg / ml, the survival rate was reduced to 30%, and when it exceeded 200 μg / ml, the cells were almost inactive ( Such as figure 1 shown). This shows tha...
Embodiment 2
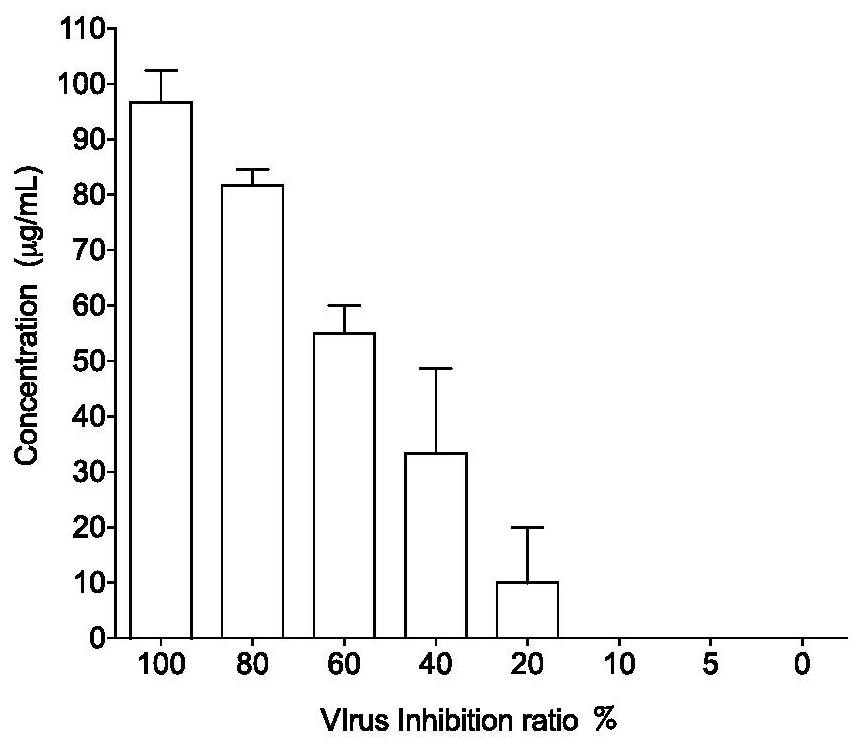
[0032] Embodiment 2 Astragaloside IV (abbreviated as AST) to the inhibition experiment of influenza virus
[0033] 1. Drugs and reagents
[0034] Astragaloside IV (Astragaloside IV), measured by HPLC with a purity of ≥98%, was purchased from AMA Copoeia Reference (40mg), and was dissolved in DMSO when used; fetal bovine serum (FBS), tetramethylthiazolium blue (MTT), bovine serum Albumin (BSA) and dimethyl sulfoxide (DMSO) were purchased from Sigma, USA; trypsin-EDTA, penicillin, and streptomycin solutions were purchased from Gibco; secondary antibodies 488-labeled goat anti-mouse IgG (H+L) was purchased from Cell Signaling Technology; DAPI nuclei luminescent dye was purchased from Beyond Biotech Co., Ltd.
[0035] 2. Viruses and Cells
[0036]The throat swab specimens collected from influenza-like cases were subjected to nucleic acid and virus isolation, detection and typing, and one-step RT-PCR method was used to amplify the HA gene and NA gene of a selected influenza B vi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com