Joint detection kit for novel influenza virus and influenza virus A/B as well as preparation method and application of joint detection kit
A technology for influenza B virus and influenza A virus, which is applied in the field of medical testing, can solve the problems of increasing the burden on patients of medical staff infection, the inability of sample extracts to replace each other, and patients being prone to missed detection, etc., so as to reduce the spread of the epidemic. risk, reducing the spread of the epidemic, and the effect of short testing time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
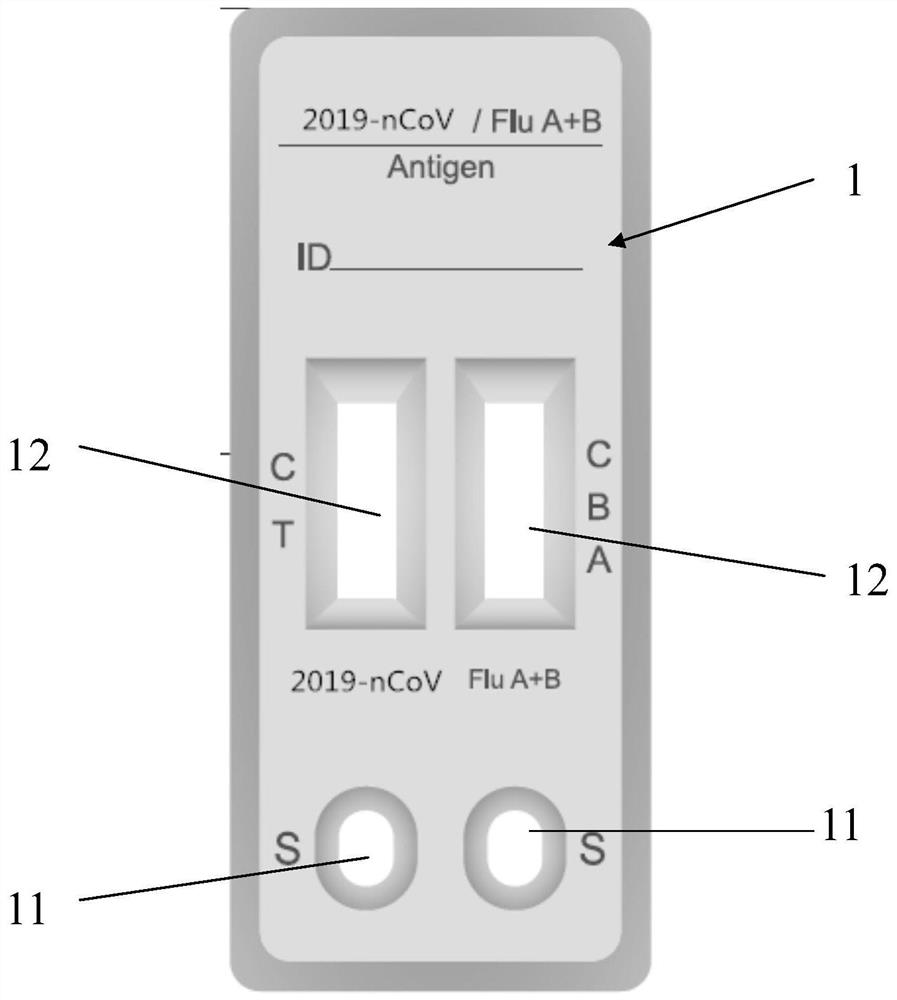
[0037] refer to Figure 1-Figure 3 As shown, the present embodiment provides a joint detection kit for new crown and influenza A and B virus antigens, which has a joint detection card (such as figure 1 As shown), the joint inspection card includes a casing 1 and a test strip, and the test strip is arranged in the casing 1. The specific setting method can be that the casing 1 includes an upper casing and a lower casing that can be fastened up and down. A groove is arranged in the lower shell, and the detection test strip is embedded in the groove. The shell 1 (upper shell) has a sample inlet 11 and an observation window 12, the sample inlet 11 is used for adding / dropping the sample to be tested, and the observation window 12 is used for observing the test result.
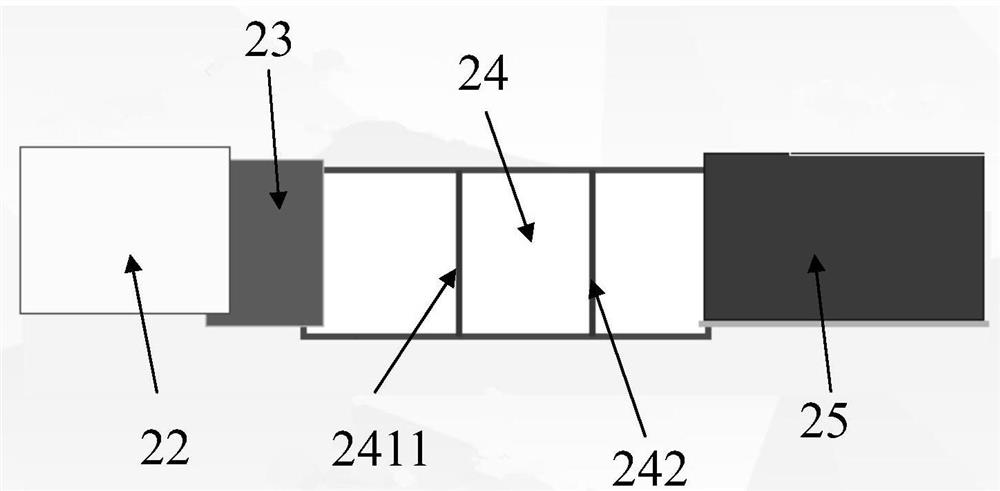
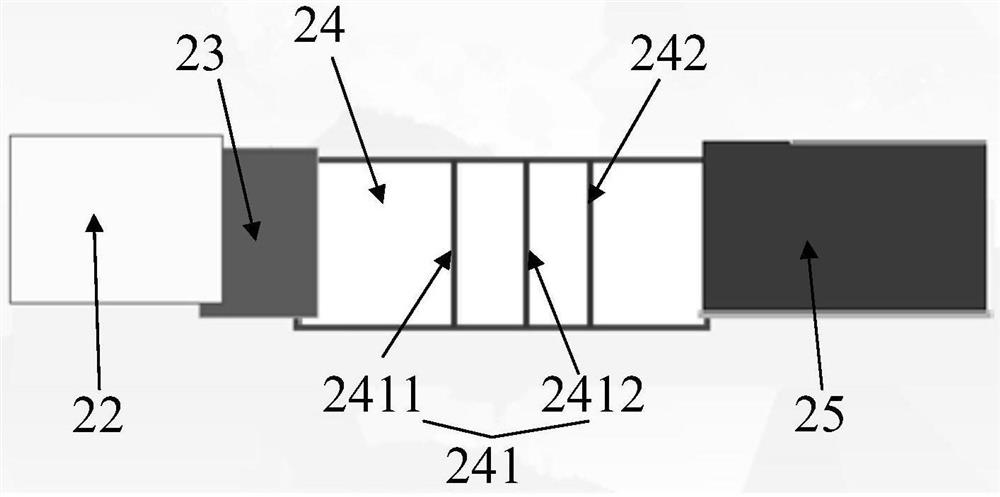
[0038] read on figure 2 and image 3As shown, the detection test strip includes a bottom plate, a sample pad 22, a binding pad 23, a detection pad 24, and an absorption pad 25. Along the chromatography direction,...
Embodiment 2
[0054] This embodiment provides a joint detection kit for new crown and influenza A and B virus antigens. The detection kit has the joint detection card described in Example 1, and also includes a sample extract, which contains 50 mM (mM is mmol / L) (final concentration) of PB (Phosphate Buffer), mass concentration 0.5% (mass final concentration) Tritonx-100, 0.1% (mass final concentration) Proclin300 and 1% (mass final concentration) NaCl, the The above-mentioned final concentration refers to the concentration of each component in the final sample extract.
[0055] The sample extract can be used to process the new crown sample and influenza A and B samples at the same time. The processed samples are tested with the new crown and influenza A and B virus antigen joint detection kit. The test results of the three items can be obtained within 30 minutes of the whole process, which is fast, efficient and effective. It helps medical staff to obtain results in a timely manner and mak...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com