Data analysis method and device
A data analysis and data technology, applied in the field of data analysis, can solve the problems of not fully reflecting the distribution characteristics of clinical trial data, and not being able to accurately evaluate the reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
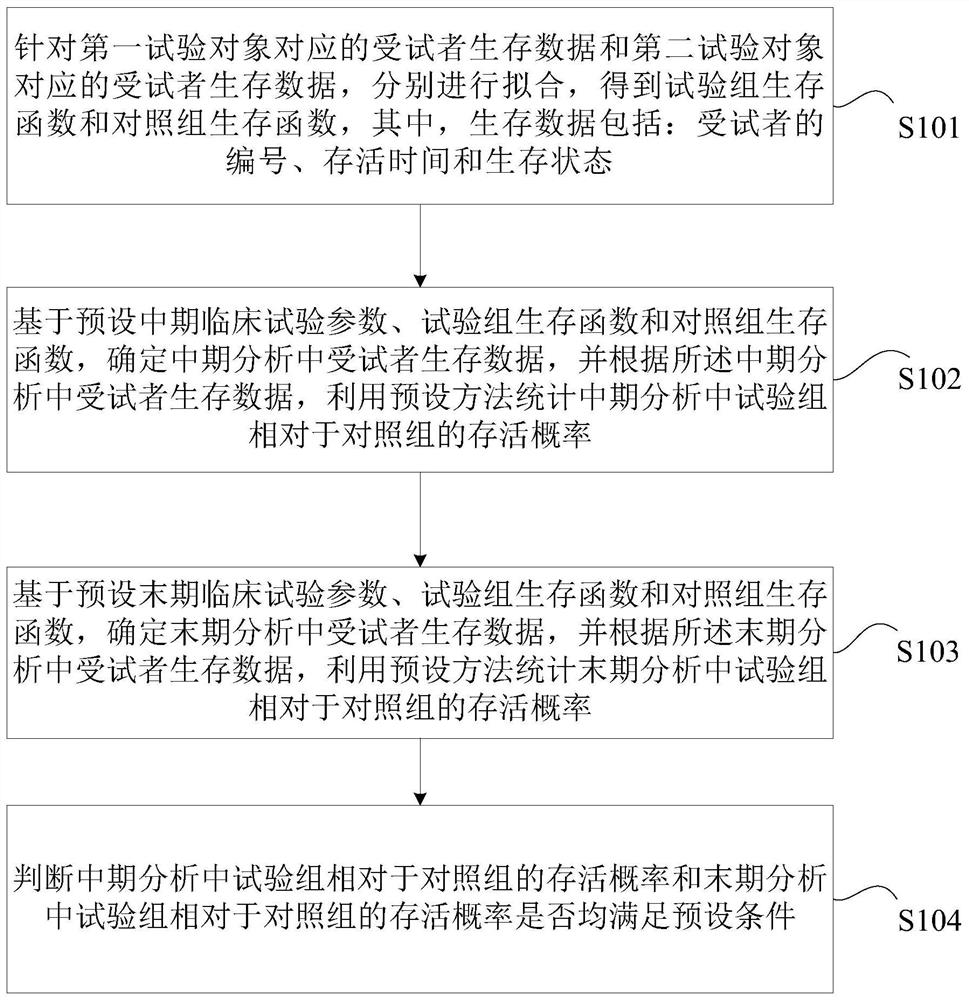
[0026] The principle and spirit of the present invention will be described below with reference to several exemplary embodiments. It should be understood that these embodiments are given only to enable those skilled in the art to better understand and implement the present invention, rather than to limit the scope of the present invention in any way. Rather, these embodiments are provided so that this disclosure will be thorough and complete, and will fully convey the scope of the invention to those skilled in the art.
[0027] Explain in detail the following industry terms that may appear.
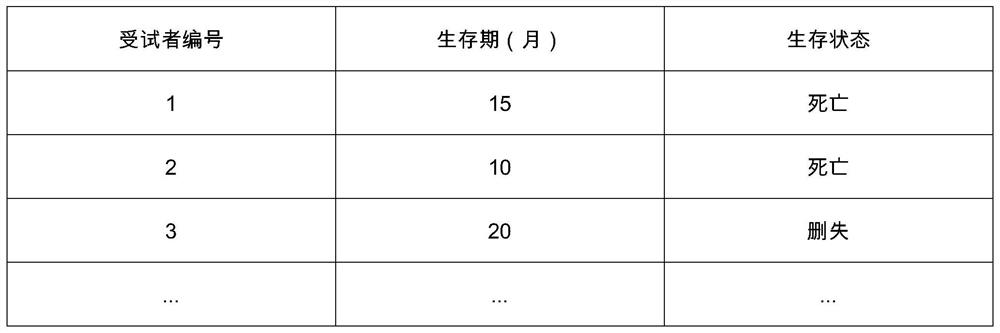
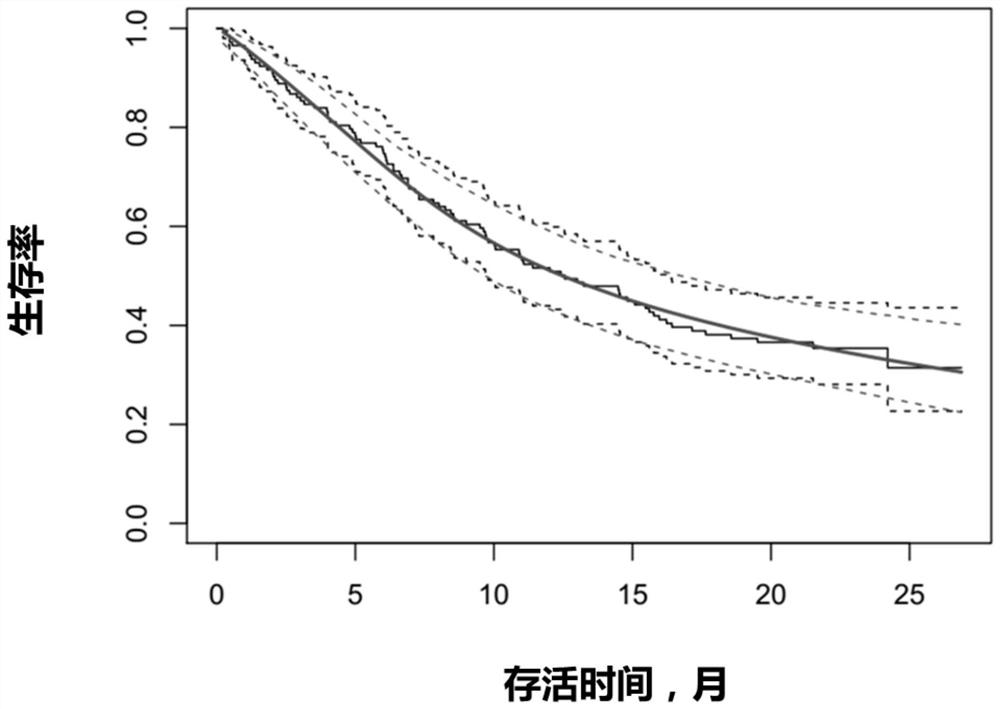
[0028] Clinical trials: Clinical trials are the gold standard for evaluating the effectiveness and safety of drugs and therapies. By means of randomized grouping, the characteristics of the subjects in the test group and the control group are balanced and comparable, and the difference in outcomes between the test group and the control group can be considered to be entirely attributable ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com