Anti-il1rap antibodies and methods of use thereof
An antibody, HVR-L1 technology, applied in chemical instruments and methods, antibodies, anti-inflammatory agents, etc., can solve the problem of lack of inhibitory effect on signal transduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0311] Various features and embodiments of the present disclosure are described in the following representative examples, which are intended to be illustrative and not restrictive. Those skilled in the art will readily appreciate that these specific examples are merely illustrative of the invention, as more fully described in the claims that follow. Each embodiment and feature described in this application should be understood to be interchangeable and combinable with each embodiment contained therein.
example 1
[0312] Example 1: Production of IL1RAP polypeptide
[0313] This example illustrates the preparation of various IL1RAP polypeptide constructs for use as antigens to elicit and screen the anti-IL1RAP antibodies of the present disclosure.
[0314] Based on structural information in Wang et al., Nature Immunology, 11:905-912 (2010), the extracellular domain of hu-IL1RAP was recombinantly purified to full-length and individual domains (ie, D1 , D2, D3). Amino acid sequence boundaries for the expression constructs are provided in Table 1 above and the accompanying Sequence Listing. All recombinant IL1RAP polypeptide constructs have the following "GGGS-V5-6XHis" C-terminal tag for purification and detection: GGGSGKPIPNPLLGLDSTHHHHHH (SEQ ID NO: 319). For purification and detection, the individual domain constructs (eg D1, D1D2, D3) have the following "GGGS-V5-Avi-6XHis" C-terminal tag (also "Avi" tag): GGGSGKPIPNPLLGLDSTGLNDIFEAQKIEWHEHHHHHH (SEQ ID NO: 320 ).
[0315] The IL1RA...
example 2
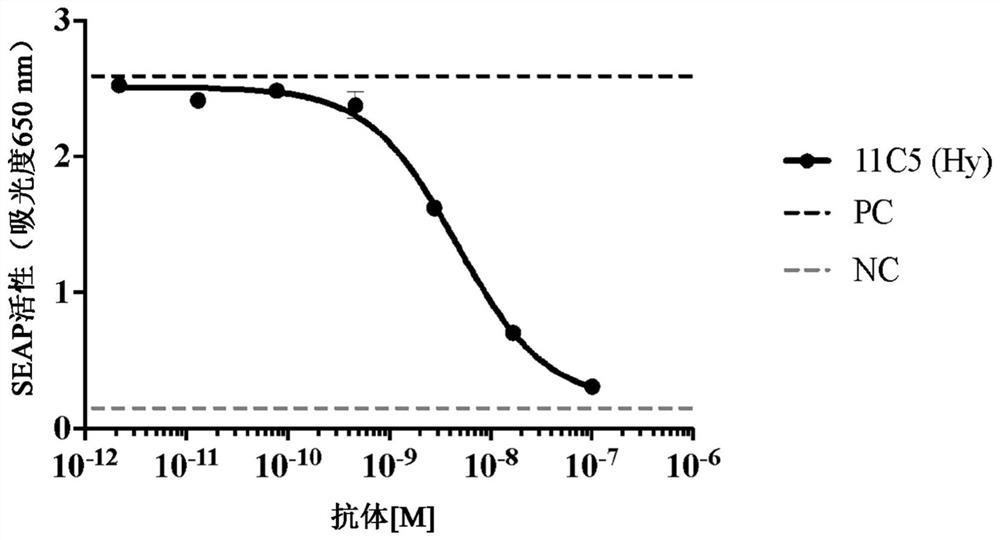
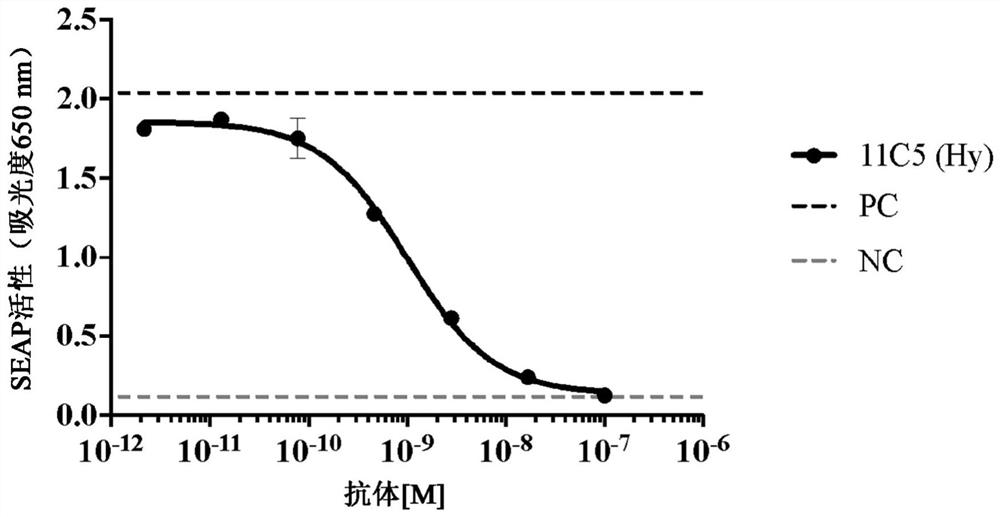
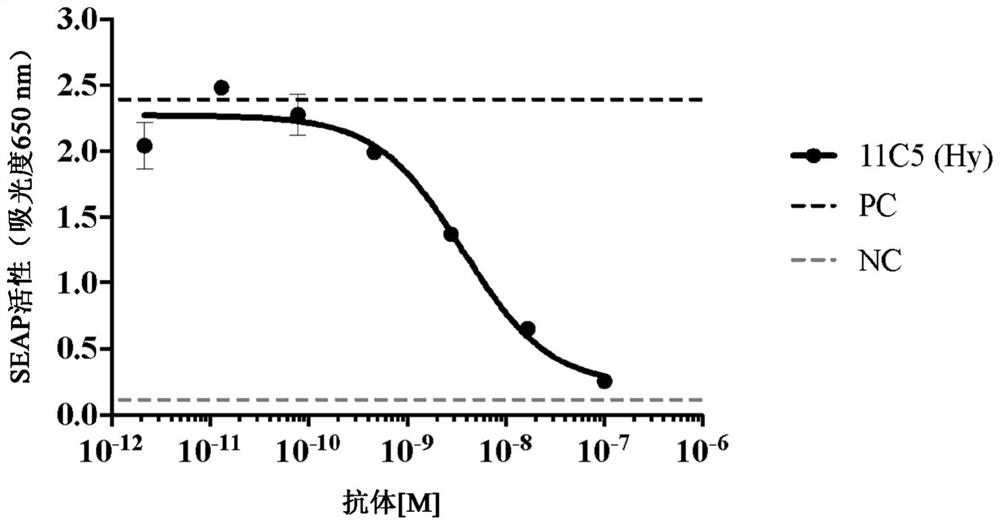
[0316] Example 2: Generation of anti-human IL1RAP antibodies using hybridoma method, screening and selection for further characterization.
[0317] This example illustrates methods for producing anti-hu-IL1RAP antibodies using mouse hybridoma technology and methods for screening and selecting antibodies for further characterization.
[0318] Immunization and fusion : Balb / c, Swiss Webster and SJ / were immunized with the hu-M-IL1RAP polypeptide construct of SEQ ID NO: 3 (see Table 1 ) comprising the membrane-formed extracellular domain of hu-IL1RAP using 50 μg / mouse L mice. Following the primary immunization, subsequent booster immunizations were administered with the following 3 different IL1RAP immunogens at the schedule and duration required to induce appropriate titers of anti-IL1RAP antibodies in mice: SEQ ID NO: 3 at 25 μg / mouse (hu-M-IL1RAP), 25 μg / mouse of SEQ ID NO:9 (mo-M-IL1RAP), or 25 μg / mouse of SEQ ID NO:6 (hu-M-IL1RAP-D3) peptide. Adjuvant Magic Mouse (Creat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com