Salts of substituted pyrrolopyrimidine cdk inhibitors and their crystallization and use
A crystallization and solvent technology, applied in the field of medicinal chemistry, can solve the problems of difficult to meet industrial production, affecting drug stability, difficult to store, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
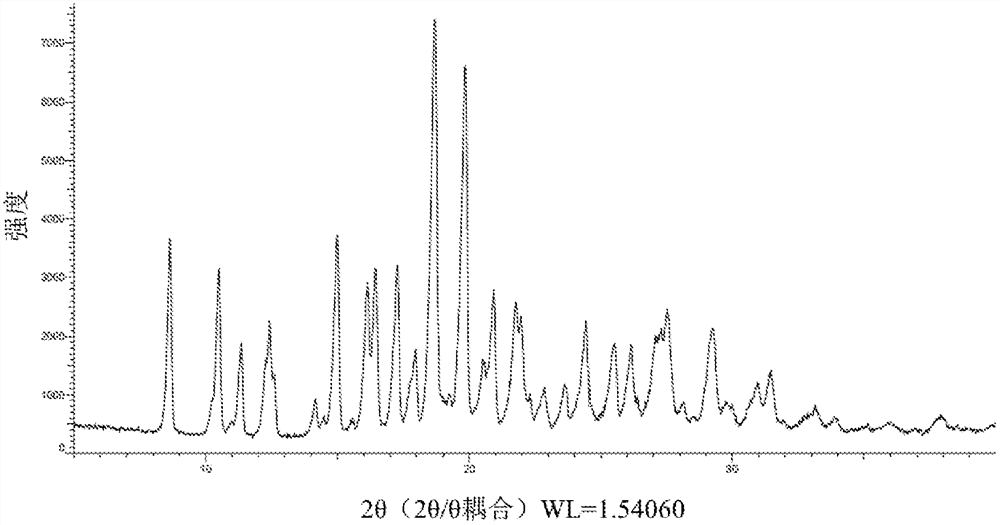
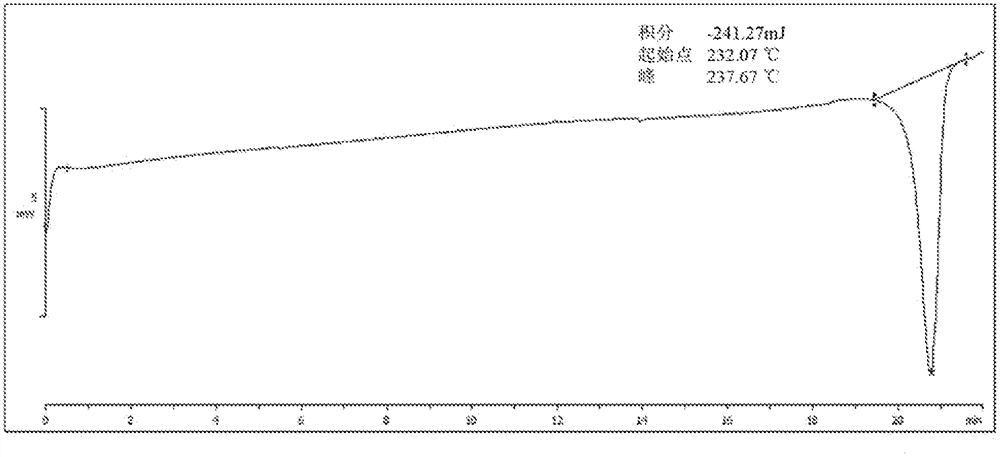
Image
Examples
preparation example Construction
[0048] In another aspect, the present application provides a method for preparing crystals of the compound of formula II, said method comprising the step of precipitating the compound of formula II from a solvent.
[0049] In some embodiments, the method for preparing crystals of the compound of formula II comprises precipitation of the compound of formula II from a solvent selected from methanol, ethanol, isopropanol, N-methylpyrrolidone or dimethyl sulfoxide. In some embodiments, the solvent is ethanol.
[0050] In some embodiments, the preparation method of the crystal of the compound of formula II comprises the following steps:
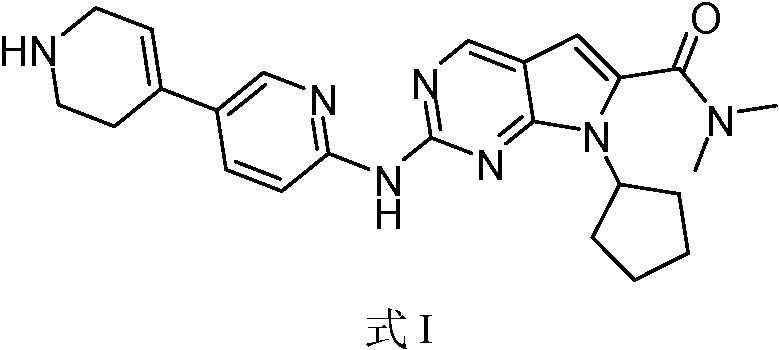
[0051]
[0052] 1) maleic acid reacts with a compound of formula I in a solvent;
[0053] 2) Precipitation of solid.
[0054] In some embodiments, step 1) is: mixing maleic acid with a solvent, mixing the compound of formula I with a solvent, and reacting the two mixtures formed above.
[0055] In some embodiments, the molar ratio of maleic ...
Embodiment 1
[0081] The preparation of embodiment 1 formula I compound
[0082]
[0083] The product obtained by referring to the preparation method of Example 6 in WO2017162215 was dissolved in heated ethanol, the temperature was lowered to precipitate a solid, filtered, and the filter cake was dried under reduced pressure to obtain crystals of the compound of formula I-1 (melting point<210°C).
[0084]Add the above-mentioned compound of formula I-1 (2.26Kg) into anhydrous methanol (22.6L), stir and dissolve at 10-30°C; add 2 mol / L sodium hydroxide aqueous solution dropwise, adjust the pH to 9-10, and drop , stirred for more than 1 h; filtered, the filter cake was washed with purified water until the pH of the washing solution was about 7, and the wet product was dried under reduced pressure at 45-55 °C for more than 8 h to constant weight to obtain crystals of the compound of formula I (1.71Kg). HRMS (ESI+, [M+H]+) m / z 432.2511, melting point 215-217°C.
[0085] The preparation of em...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap