Application of trilobatin in preparation of medicine for preventing and/or treating non-alcoholic fatty liver disease
A technology for non-alcoholic fatty liver disease, which is applied in the application field of preparing drugs for preventing and/or treating non-alcoholic fatty liver disease, and can solve the problems that there is no relevant research on the effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
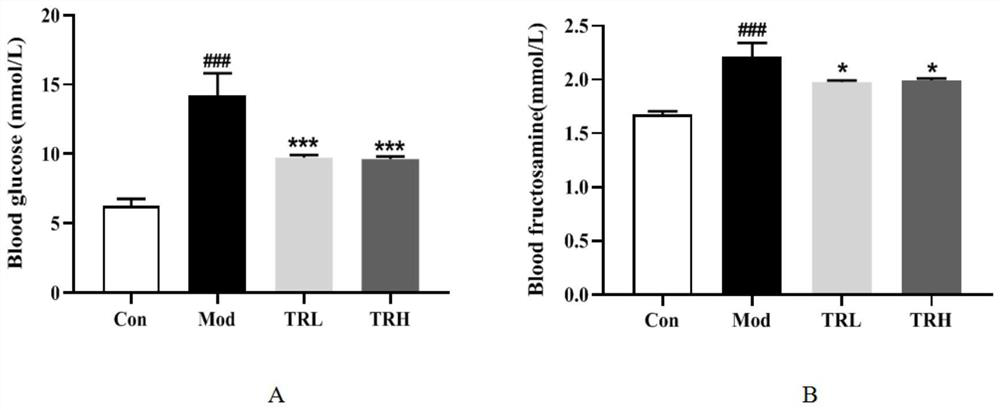
[0050] Example 1 Effect of Trilobatin on Glucose Metabolism in Nonalcoholic Fatty Liver Mice
[0051] 1. Experimental method
[0052] Immediately after the end of the experiment, the blood was drawn from the heart, and sodium heparin was added for anticoagulation. After the collected blood was left for 30 minutes, it was centrifuged at 4000rpm / min for 10 minutes, and the supernatant was collected and packed into EP tubes, and stored in a -80°C refrigerator.
[0053] Plasma was taken, and fasting blood glucose (Glu) and fructosamine (Fru) were detected using an automatic biochemical analyzer.
[0054] 2. Experimental results
[0055] Glucose metabolism results such as figure 1 As shown, compared with the normal group, Glu and Fru of the mice in the model group were significantly increased (Pfigure 1 -A, Pfigure 1 -B, P<0.05). The results showed that trilobatin could improve blood glucose metabolism disorder in non-alcoholic fatty liver mice.
Embodiment 2 3
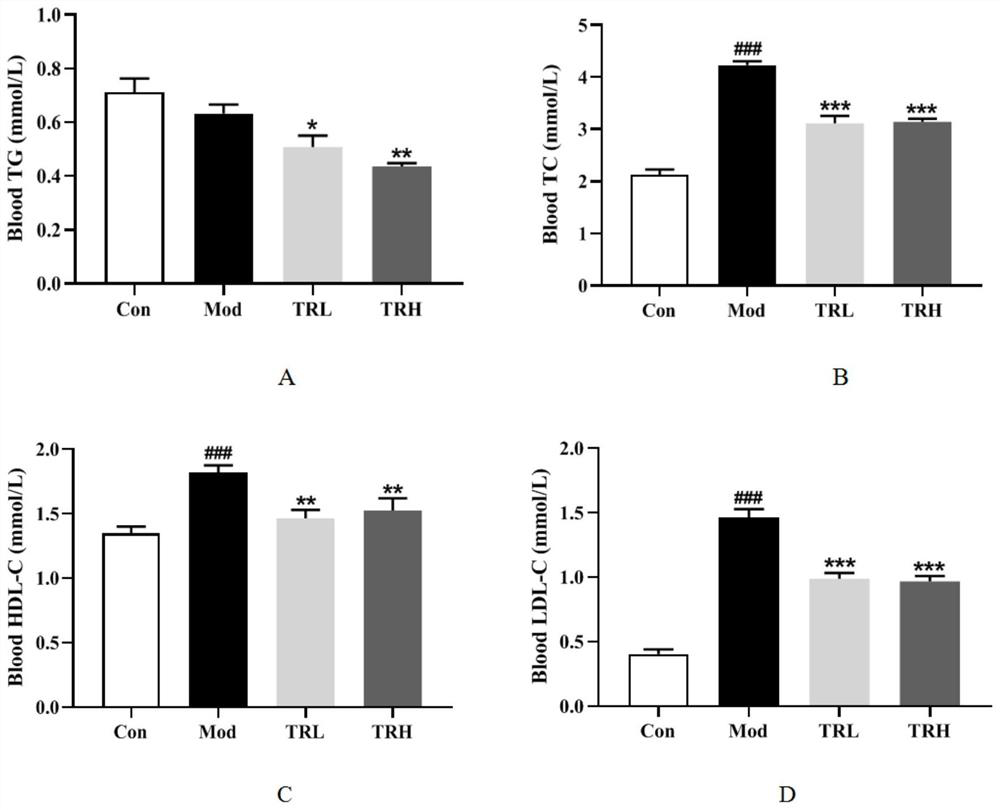
[0056] Example 2 Effect of Trilobatin on Lipid Metabolism in Nonalcoholic Fatty Liver Mice
[0057] 1. Experimental method
[0058] Plasma was collected, and the levels of triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were detected using an automatic biochemical analyzer.
[0059] 2. Experimental results
[0060] Lipid metabolism results such as figure 2 As shown, compared with the normal group, the TC, LDL-C, and HDL-C contents of the mice in the model group were all significantly increased ( figure 2 -B, figure 2 -C, figure 2 -D, P figure 2 -A, P>0.05); compared with the model group, the trilobatin low and high dose groups could significantly reduce the contents of TC, LDL-C and HDL-C in mouse plasma ( figure 2 -B, figure 2 -C, figure 2 -D, P figure 2 -A, P<0.05, P<0.01), the results show that trilobatin can improve the abnormal blood lipid metabolism in non-alcoholic fat...
Embodiment 3 3
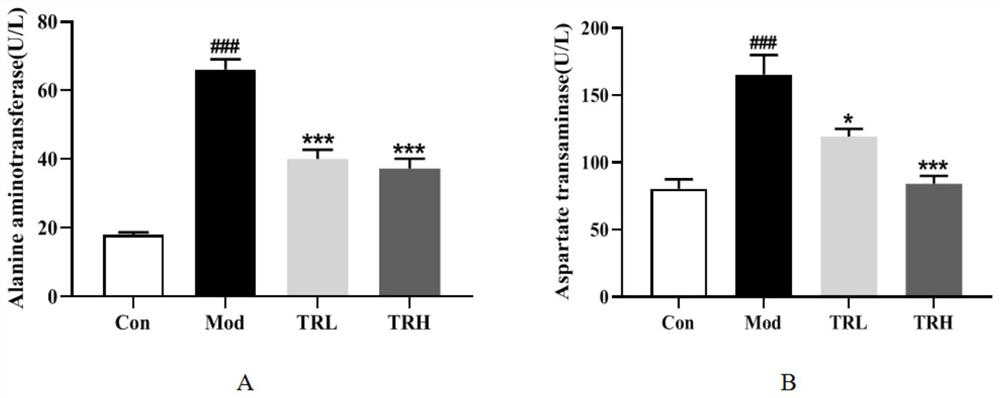
[0061] Example 3 Effect of trilobatin on liver tissue ALT and AST of nonalcoholic fatty liver mice
[0062] 1. Experimental method
[0063] Plasma was taken, and the activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the plasma were detected using an automatic biochemical analyzer.
[0064] 2. Experimental results
[0065] Transaminases are involved in the whole process of the liver, and an important sign of liver problems is elevated transaminases. ALT mainly exists in the liver cytoplasm, and AST mainly exists in the mitochondria of liver cells. The two most sensitive enzymes of liver cells are ALT and AST. When the liver cells are damaged, the activities of ALT and AST increase, which can reflect the damage of liver cells. degree of size. The result is as image 3 As shown, compared with the blank group, the levels of ALT and AST in the liver tissue of the mice in the model group were significantly increased ( image 3 -A, image 3 -B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com