A kind of synthetic method of 2-amino-6-chloropurine
A synthetic method, the technology of chloropurine, applied in the direction of organic chemistry, etc., can solve the problems of odor generation, unfavorable industrial application, environmental pollution, etc., and achieve the effects of reducing use, facilitating large-scale process production, and reducing emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
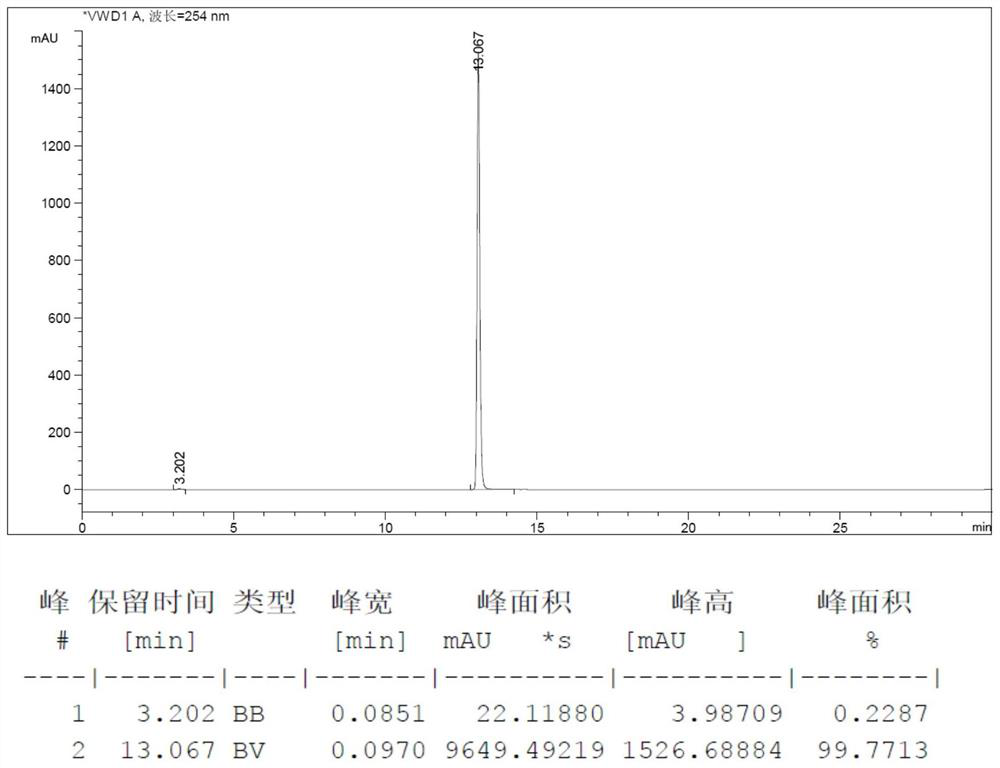
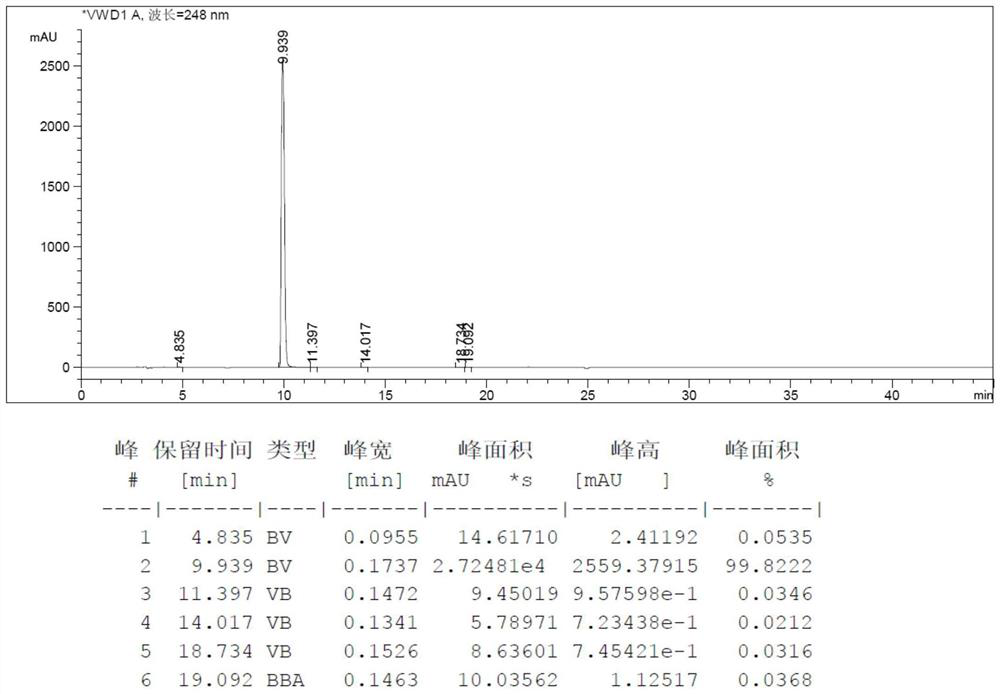
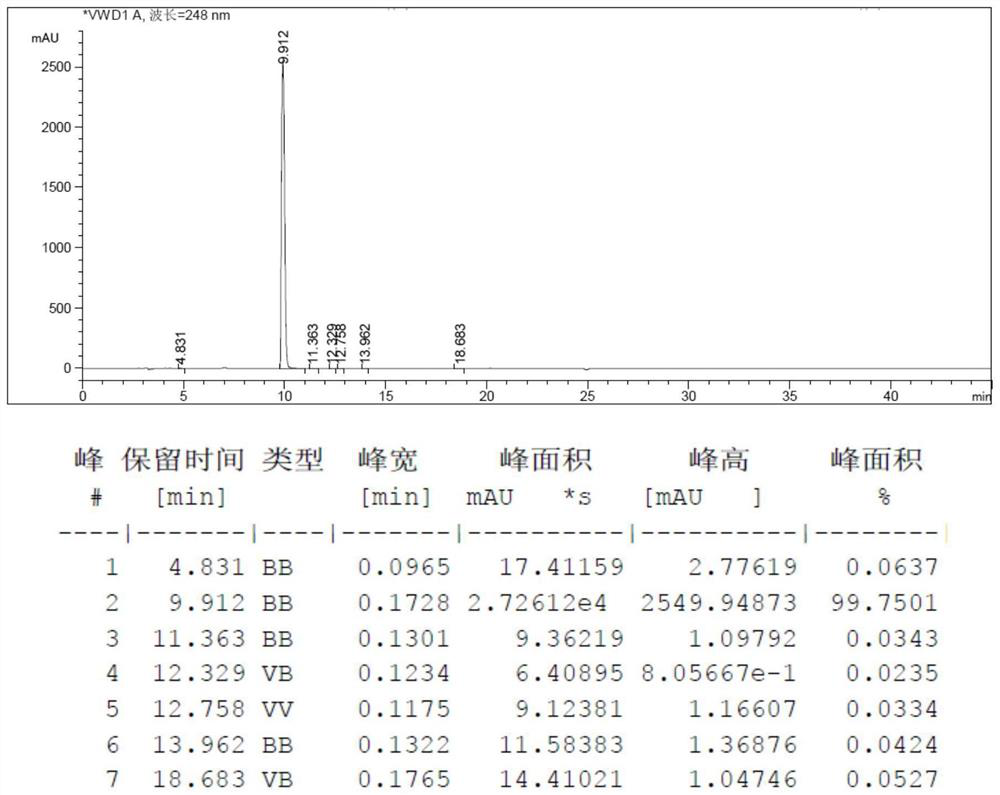
Image
Examples
Embodiment 1
[0034] The synthetic method of 2-acetylamino-6 hydroxypurine
[0035] The preparation method of 2-acetylamino-6 hydroxypurine provided in this embodiment comprises the following steps:
[0036] Step 1, guanine (10g, 66.2mmol, 1eq), acetic anhydride (100g, 0.980mol) and tetrabutylammonium bromide (0.47g, 1.46mmol, 0.022eq) were added to the reaction vessel, and the temperature was raised to 110°C , stirred and reacted for 2 hours, then lowered the temperature to 25°C, stood still for 2h, solid precipitated, filtered, separated the solid from the filtrate, and dried the solid at 50°C to obtain 15.2g of 2-acetylamino-9-acetyl-6 Hydroxypurine, the single-step yield is 97.6%, the filtrate is concentrated under reduced pressure at 85°C, and can be recovered and used mechanically after separating acetic acid;
[0037] Step 2, dissolving the 2-acetylamino-9-acetyl-6-hydroxypurine obtained in step 1 in 50 mL of dichloromethane (DCM), adding 50 mL of saturated aqueous sodium bicarbonat...
Embodiment 2
[0041] Screening of Catalysts in the Synthesis of 2-Acetamido-6-Hydroxypurine
[0042] In this example, the acetylation catalyst in the preparation method of 2-acetylamino-6-hydroxypurine was screened. The specific experimental operation was the same as in Example 1, the only difference being that the acylation catalyst used was different or the amount was different. The specific results were as follows Table 1 shows.
[0043] Table 1 Catalyst Screening Table
[0044]
[0045]
[0046] * The yield is the yield of 2-acetylamino-9-acetyl-6-hydroxypurine synthesized in step 1, and the reaction of step 2 was not carried out due to the low yield.
[0047] As shown in the table above, some common acylation catalysts can catalyze the diacetylation reaction of guanine to a certain extent, but only quaternary ammonium salt catalysts can basically complete the reaction within 2 hours, achieving a yield of more than 85%. , wherein, among various quaternary ammonium salt catalyst...
Embodiment 3
[0049] The synthetic method of 2-amino-6-chloropurine
[0050] The present embodiment provides a kind of preparation method of 2-amino-6-chloropurine, comprises the steps:
[0051] Step 1, add 2-acetamido-6 hydroxypurine (50g, 0.26mol, 1eq, loss on drying 1.2%), hexachloroacetone (82.6g, 0.31mol, 1.2eq), N-methylpiperidine (0.51g, 0.0052mol, 0.02eq) and thionyl chloride (400mL), heated to reflux at 85°C for 24h, distilled off thionyl chloride, added 300mL with a mass fraction of 10 % sodium hydroxide aqueous solution, stir at 25°C for 6h, use 1mol / L hydrochloric acid aqueous solution to adjust the pH value to 7.0-7.5, add 300mL water, raise the temperature to 60°C, keep warm for 1h, cool to 25°C, stir for 10 Minutes, a solid is precipitated, centrifuged to obtain the crude product of 2-amino-6-chloropurine;
[0052] Step 2, adding the crude 2-amino-6-chloropurine obtained in step 1 to a 7% sodium hydroxide aqueous solution, adding 5 g of activated carbon for decolorization for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com