A kind of chiral chromatography packing of substituted polyalkyne grafted divinylbenzene microspheres and preparation method thereof
A technology of divinylbenzene and chiral chromatography, which is applied in the field of chiral chromatography packing and its preparation, can solve problems such as poor stability, and achieve the effect of improving stability and avoiding damage to the helical structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
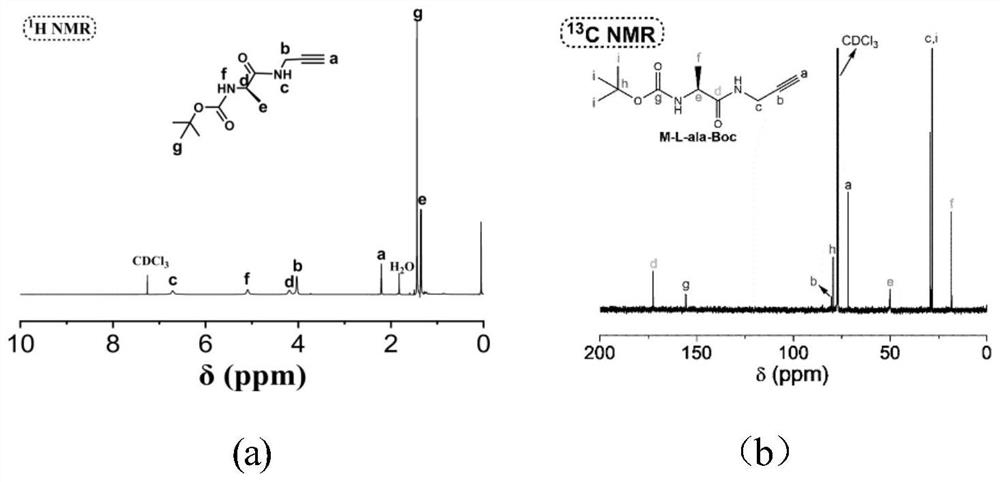
[0057] The first step was to prepare M1 as follows: 1.90 g of N-Boc-alanine ((N-tert-butoxycarbonyl-alanine; CAS: 15761-38-3) was dissolved in 40 mL of THF, followed by the addition of 1.3 mL of Isobutyl chloroformate and 1.1 mL of N-methylmorpholine were reacted at 30 °C for 15 min, then 0.7 mL of propargylamine was added to the flask, reacted in a water bath at 30 °C for 4 h, and the resulting precipitate was removed by filtration; the filtrate was washed with ethyl acetate Ester extraction, washed three times with 30 mL of hydrochloric acid solution (2M), twice with 30 mL of saturated sodium bicarbonate solution, and once with 80 mL of deionized water, then dried with anhydrous magnesium sulfate, distilled under reduced pressure, in THF / n-hexane Purify by cooling and crystallization in a system with a volume ratio of 1 / 6. After 5 hours, filter and dry at 30°C for 24h-48h to obtain M1 solid product with a yield of 80%.
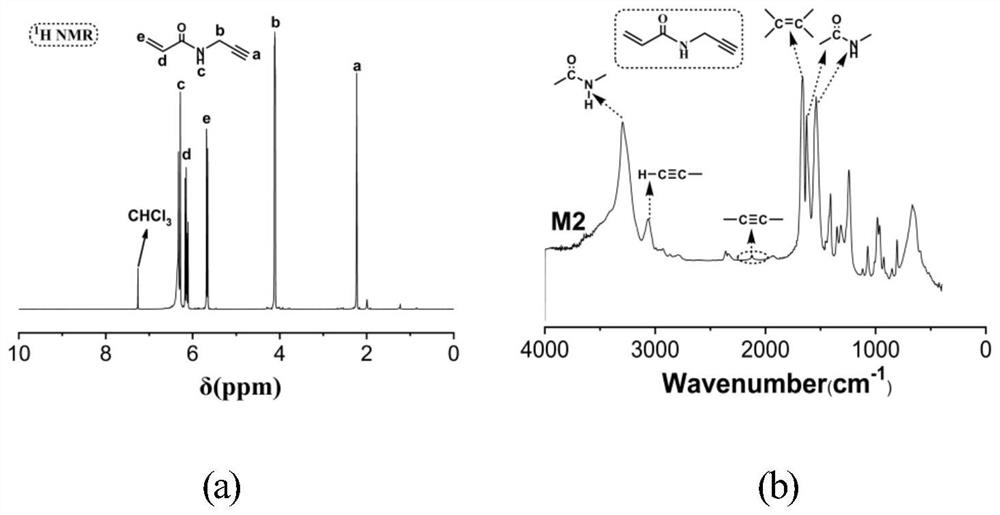
[0058] The second step to prepare M2 is as follows: di...
Embodiment 2
[0075] The first step is to prepare the substituted alkyne monomer M1, and the specific method is as in Example 1.
[0076] The second step is to prepare the substituted alkyne monomer M2, and the specific method is as in Example 1.
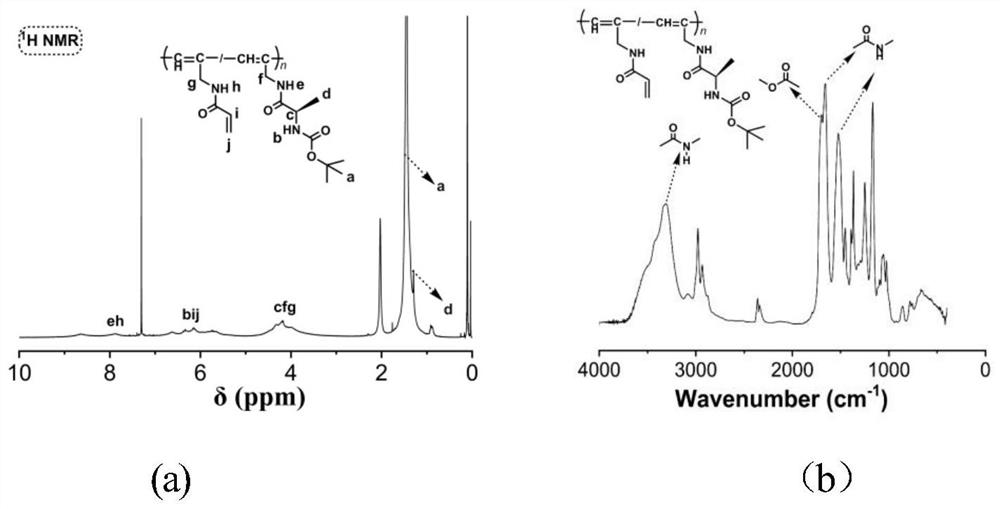
[0077] The third step is to prepare the substituted polyalkyne P-M1-co-M2-Boc, as follows: add 0.113g of M1, 0.055g of M2, 0.005g of (nbd)Rh to the test tube + B - (C 6 H 5 ) 4 catalyst, and then the test tube was repeatedly vacuumed and argon treated for a total of three times. Then 5ml of THF was added under argon protection, the test tube was sonicated until the solid was dissolved, then the test tube was heated in a water bath at 30°C, and a pale red solution was obtained after polymerization for 6 hours. The obtained solution was added dropwise to n-hexane for precipitation and filtered. After drying, precipitation, filtration and drying were performed twice in a system with a volume ratio of THF / n-hexane of 1 / 6 to obtain yellow P-M1-co...
Embodiment 3
[0084] The first step is to prepare the substituted alkyne monomer M1, and the specific method is as in Example 1.
[0085] The second step is to prepare the substituted alkyne monomer M2, and the specific method is as in Example 1.
[0086] The third step is to prepare the substituted polyalkyne P-M1-co-M2-Boc, as follows: add 0.136g of M1, 0.044g of M2, 0.005g of (nbd)Rh to the test tube + B - (C 6 H 5 ) 4 catalyst, and then the test tube was repeatedly vacuumed and argon treated for a total of three times. Then 5ml of THF was added under argon protection, the test tube was sonicated until the solid was dissolved, then the test tube was heated in a water bath at 30°C, and a pale red solution was obtained after polymerization for 6 hours. The obtained solution was added dropwise to n-hexane for precipitation and filtered. After drying, precipitation, filtration and drying were carried out twice in a system with a volume ratio of THF / n-hexane of 1 / 6 to obtain yellow P-M1-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com