Electronic medical record diagnosis and treatment quality control method based on clinical diagnosis and treatment guide
An electronic medical record and quality control technology, applied in the field of medical system informatization, can solve the problems of lagging medical process, dependence, and few quality control practitioners.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
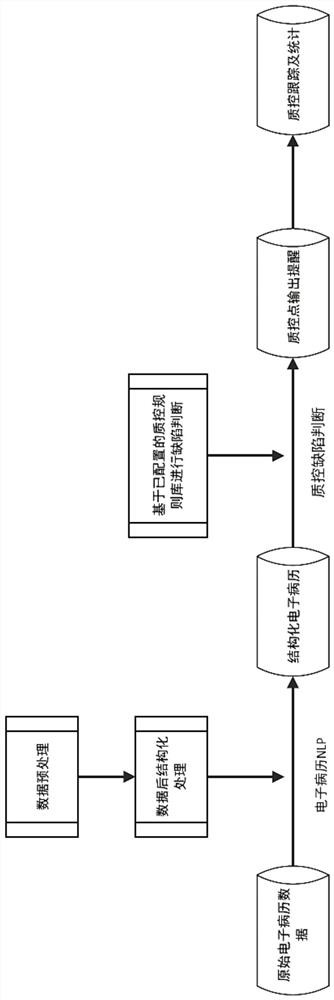
[0074] 1. Integrated display of medical records Collect and display patient diagnosis and treatment information in each system, including electronic medical records, tests, inspections, doctor's orders and other information.
[0075] 2. Quality control window reminder The system automatically processes and judges patient data in real time. If there is any behavior that does not meet the guidelines in the patient's medical record, the system will display the defective quality control points in the quality control window. The quality control window includes modules such as diagnosis, inspection, medication, and treatment.
[0076] 3. Defect location The system can locate the error in the original text of the medical record for each defect point, which is convenient for doctors to quickly find the problem.
[0077] 4. Scoring of quality control points The system provides weight setting and score calculation functions for each quality control point. The quality control window dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com