A kind of rare earth metal material and its preparation method and application
A rare earth metal material and reaction technology, which is applied in the direction of luminescent materials, analytical materials, and material excitation analysis, etc., can solve the problems of demanding test samples, fixed test locations and durations, high equipment procurement and maintenance costs, and reduce test steps. The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The invention provides a preparation method of rare earth metal material, comprising the following steps:
[0053] The rare earth metal material is obtained by mixing nitrate, aminotriacetic acid and a solvent and performing a coordination reaction.
[0054] In the present invention, the nitrate is preferably neodymium nitrate hexahydrate, terbium nitrate hexahydrate or europium nitrate hexahydrate.
[0055] In the present invention, the molar ratio of the nitrate to aminotriacetic acid is preferably 1-2:1-2, more preferably 1.2-1.8:1.2-1.8, more preferably 1.4-1.6:1.4-1.6.
[0056] In the present invention, the solvent preferably contains water and N,N-dimethylformamide.
[0057] In the present invention, the volume ratio of the water and N,N-dimethylformamide is preferably 1-2:1-2, more preferably 1.2-1.8:1.2-1.8, more preferably 1.4-1.6:1.4 ~1.6.
[0058] In the present invention, the specific polarity prepared by mixing N,N-dimethylformamide and water in proporti...
Embodiment 1
[0072] Take 0.2 mmol of neodymium nitrate hexahydrate, 0.2 mmol of aminotriacetic acid, and 20 mL of solvent; the volume ratio of water and N,N-dimethylformamide in the solvent is 1:1;
[0073] The rare earth metal materials were prepared according to the following method: the neodymium nitrate hexahydrate, aminotriacetic acid and the solvent were mixed by ultrasonic at 40KHz and 25°C for 30min, and then the mixed system was coordinated at 90°C for 72h. After the completion of the coordination reaction , the reaction solution was naturally cooled to room temperature, the impurities were filtered, and the light purple rod-shaped crystal obtained by drying at 25° C. for 48 hours was the rare earth metal material, and was denoted as complex 1.
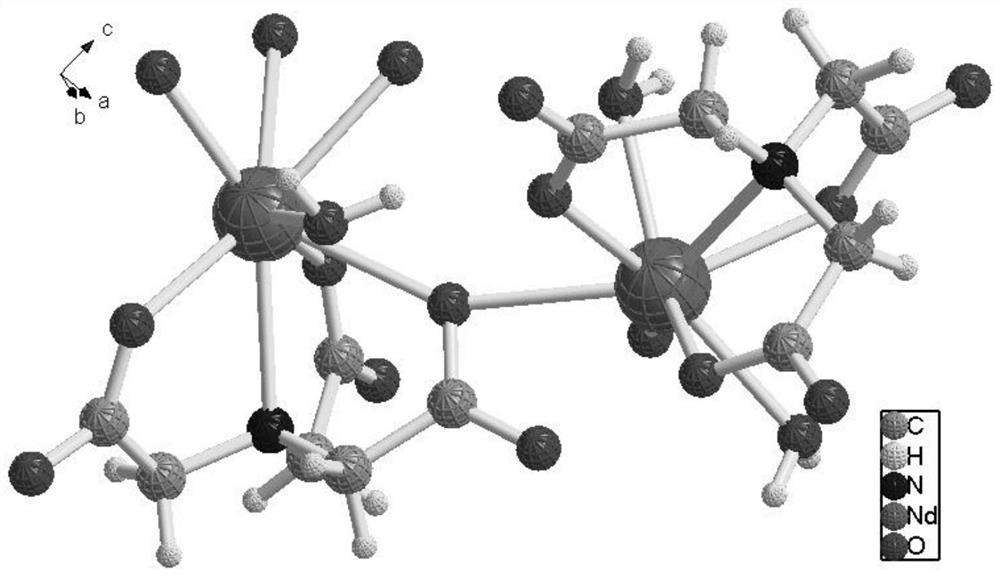
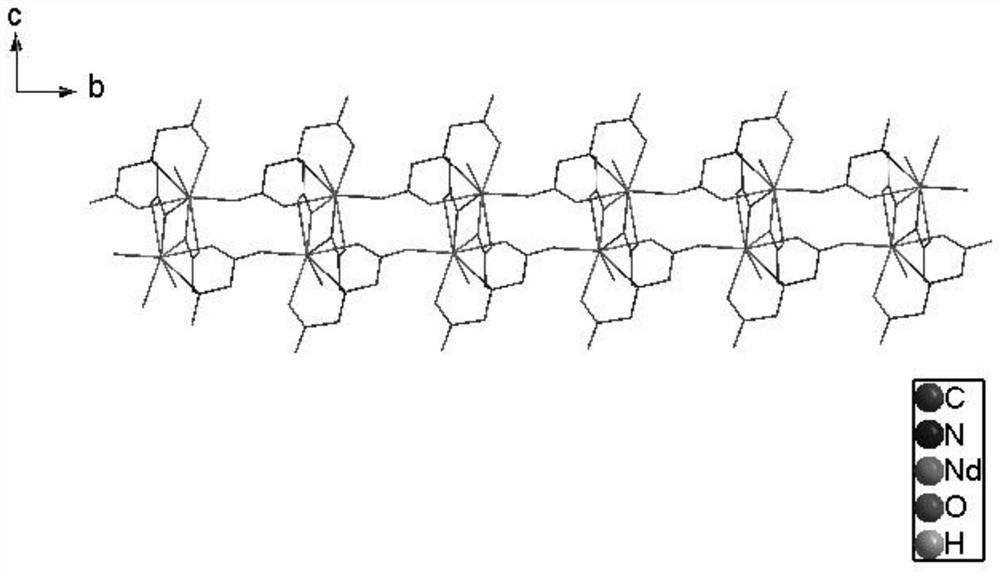
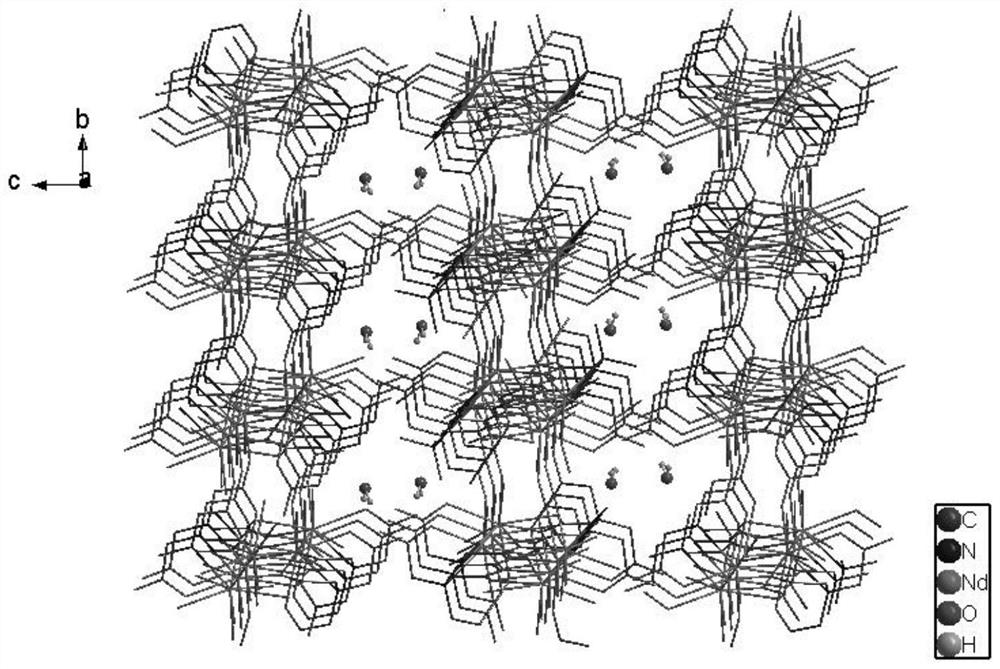
[0074] The three-dimensional crystal structure of complex 1 is as figure 1 shown, from figure 1 It can be seen that the two metallic neodymiums in the complex are connected by four acetate groups of the shared ligand nitrilotriacetic aci...
Embodiment 2
[0084] Take 2mol terbium nitrate hexahydrate, 2mol aminotriacetic acid, 200L solvent; the volume ratio of water and N,N-dimethylformamide in the solvent is 1:1;
[0085] The rare earth metal materials were prepared according to the following method: the terbium nitrate hexahydrate, aminotriacetic acid and the solvent were mixed by ultrasonic at 30KHz and 28°C for 40min, and then the mixed system was coordinated at 130°C for 72h. After the completion of the coordination reaction , the reaction solution was naturally cooled to room temperature, the impurities were filtered, and the transparent massive particles obtained by drying at 20° C. for 56 h were the rare earth metal materials, denoted as complex 2.
[0086] The three-dimensional crystal structure of complex 2 is as Figure 11 shown, from Figure 11 It can be seen that the two metal terbium in the complex are connected by four acetate groups of the shared ligand nitrilotriacetic acid to form an assembled unit. The three...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com