Novel compounds for treatment, alleviation or prevention of disorders associated with tau aggregates
A compound and protein aggregate technology, applied in organic chemistry, drug combination, nervous system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0133]
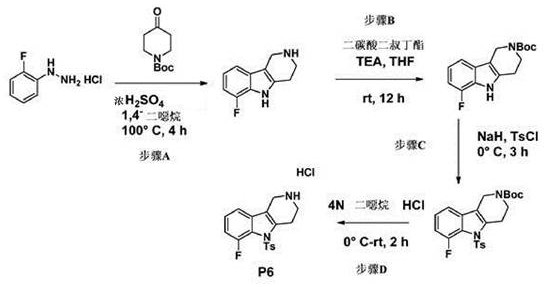
[0134] Step A
[0135] To a solution of 4-fluorophenylhydrazine (1 g, 7.9 mmol) and tert-butyl 4-oxopiperidine-1-carboxylate (1.2 g, 8.3 mmol) in 1,4-dioxane (10 mL) , add concentrated H at ice bath temperature 2 SO 4 (1 mL). The reaction mixture was then heated at 110°C for 3 hours. The reaction mixture was cooled to room temperature and the precipitate was filtered off. The solid was dissolved in water, basified with NaOH solution and extracted with DCM (dichloromethane). The organic phase was separated and washed with Na 2 SO 4 Drying and removal of solvent gave the title compound (950 mg, 59%) as a light yellow solid.
[0136]
[0137] Step B
[0138] To a solution of the title compound from Step A above (0.95 g, 4.77 mmol) in THF (tetrahydrofuran) was added di-tert-butyl dicarbonate (Boc 2 (0) (1.5 g), and the mixture was stirred overnight. After thin layer chromatography (TLC) confirmed the completion of the reaction, the solvent was removed...
preparation example 2
[0149]
[0150] Step A
[0151]Add 3-(fluorophenyl)hydrazine (1 g, 6.1 mmol) and tert-butyl 4-oxopiperidine-1-carboxylate (1.2 g, 6.1 mmol) in 1,4-dioxane (10 mL) solution, add concentrated H at 0°C 2 SO 4 (1 mL). The reaction mixture was then warmed to 25°C and heated at 110°C for 3 hours. The reaction mixture was cooled to room temperature, and the precipitate was filtered off. The solid was dissolved in water, basified with NaOH solution and extracted with dichloromethane. The organic phase was separated and washed with Na 2 SO 4 Drying and removal of solvent gave a mixture of regioisomers (0.65 g, 56%) as a light yellow solid.
[0152]
[0153] Step B
[0154] To a solution of the regioisomer mixture (0.65 g, 3.15 mmol) in THF was added di-tert-butyl dicarbonate (0.757 g, 3.47 mmol), and the mixture was stirred for 12 hours. After completion of the reaction (monitored by TLC), the solvent was concentrated under reduced pressure to obtain the crude produc...
preparation example 3
[0169]
[0170] Step A
[0171] Add (2-chloro-3-fluorophenyl)hydrazine (10g, 62.5mmol) and tert-butyl 4-oxopiperidine-1-carboxylate (12g, 62.5mmol) in 1,4-dioxane at 0°C (100 mL) was added concentrated H 2 SO 4 (10 mL). The reaction mixture was then warmed to 25°C and heated at 110°C for 3 hours. The reaction mixture was cooled to room temperature, and the precipitate was filtered off. The solid was dissolved in water, basified with NaOH solution and extracted with dichloromethane. The organic phase was separated and washed with Na 2 SO 4 Drying and removal of solvent gave the title compound (10 g, 72%) as a light yellow solid.
[0172]
[0173] Step B
[0174] To a solution of the title compound from Step A above (10 g, 44.5 mmol) in THF (100 mL) was added di-tert-butyl dicarbonate (10.5 g, 46.5 mmol), and the mixture was stirred for 12 hours. After completion of the reaction (monitored by TLC), the solvent was concentrated under reduced pressure to give c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap