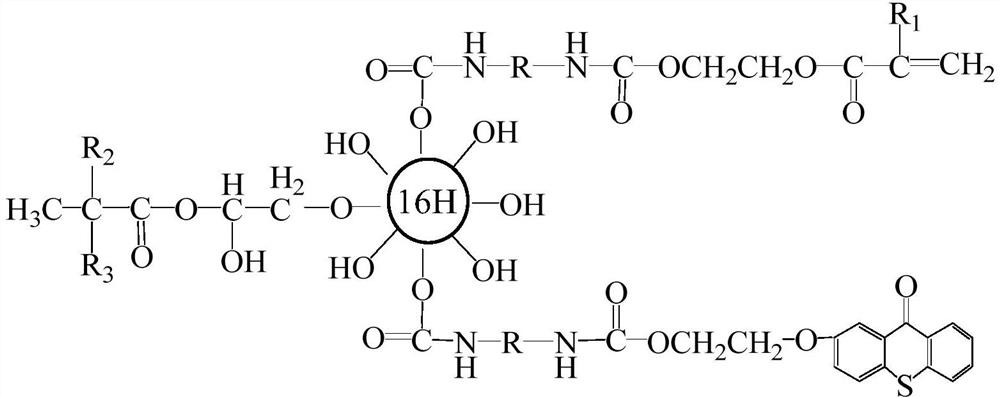
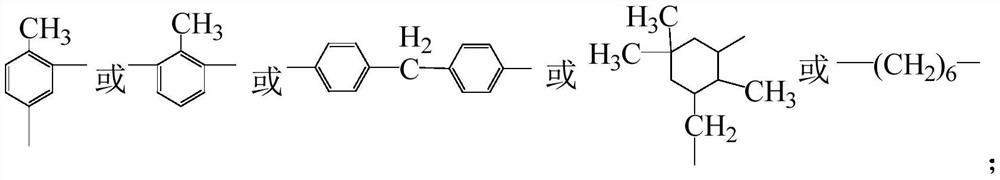
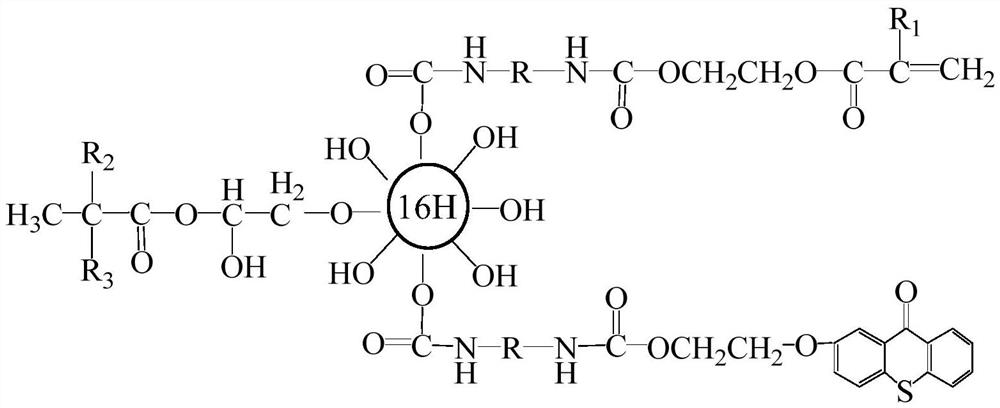
Thioxanthone photo-initiation group modified second-generation hyperbranched LED resin
A thioxanthone photo-initiating-based technology, applied in applications, coatings, inks, etc., can solve problems such as poor solubility, achieve excellent flexibility, shorten spacing, and improve photosensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Prepare the 2nd generation hyperbranched polymer HPB-2p, its step is as follows: join by 27.2 parts of pentaerythritol, 108.2 parts of 2,2-dimethylolpropionic acid, 30.0 parts of xylene, 0.55 parts of catalyst p-toluenesulfonic acid In the four-necked flask with stirrer, thermometer, adding funnel and reflux water separator, heat to 140-160°C under nitrogen protection and reflux for 4 hours under normal pressure. When the detected acid value is lower than 25mgKOH / g, add 216.4 parts of 2 , 2-Dimethylol propionic acid, 1.1 parts of p-toluenesulfonic acid and 40.0 parts of xylene, reflux at 140-160°C for 2 hours at normal pressure, then reduce the pressure to 1.2KPa for 2 hours and then stop the pressure reduction. Stop the reaction when the acid value is lower than 20mgKOH / g; evaporate xylene under reduced pressure, add 80.0 parts of acetone after cooling, dissolve completely, add 120.0 parts of toluene and stir, let stand to precipitate, filter, vacuum dry at 50°C, and ob...
Embodiment 2
[0049] Preparation of the 2nd generation hyperbranched polymer HPB-2b: Add 27.2 parts of pentaerythritol, 120.0 parts of 2,2-dimethylolbutyric acid (DMBA), 32.0 parts of xylene, 0.5 parts of catalyst p-toluenesulfonic acid into a stirring In a four-necked flask with a thermometer, a thermometer, an addition funnel, and a reflux water separator, heat it under nitrogen protection to 105-120°C under normal pressure and reflux for 4 hours. When the detected acid value is lower than 25mgKOH / g, add 240.0 parts of 2, 2-Dihydroxymethylbutyric acid, 1.1 parts of p-toluenesulfonic acid and 40.0 parts of xylene, reflux at 105-120°C for 2 hours at normal pressure, then reduce the pressure to 1.2KPa for 2 hours and then stop the pressure reduction. Stop the reaction when the acid value is lower than 20mgKOH / g; evaporate the xylene under reduced pressure, add 85.0 parts of acetone after cooling, dissolve completely, add 120.0 parts of toluene and stir, let stand to precipitate a precipitate,...
Embodiment 3
[0051] Preparation of isocyanate-acrylic acid functional monomer TDI-HEA: Add 87.0 parts of TDI-80 and 0.05 parts of DBTDL to a four-necked flask equipped with a reflux condenser, a thermometer, a dropping funnel and a stirrer, and stir to raise the temperature. Slowly add a mixture of 58.0 parts of β-hydroxyethyl acrylate, 0.1 parts of hydroquinone and 40.0 parts of acetone dropwise. After the addition is completed, the temperature is raised to 45-50°C to continue the reaction for 4 hours, and then samples are taken every 30 minutes to detect the NCO value, when the detected NCO value is half of the initial value, the reaction is stopped, and the temperature is lowered to 40°C to prepare the isocyanate-acrylic acid functional monomer TDI-HEA.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com