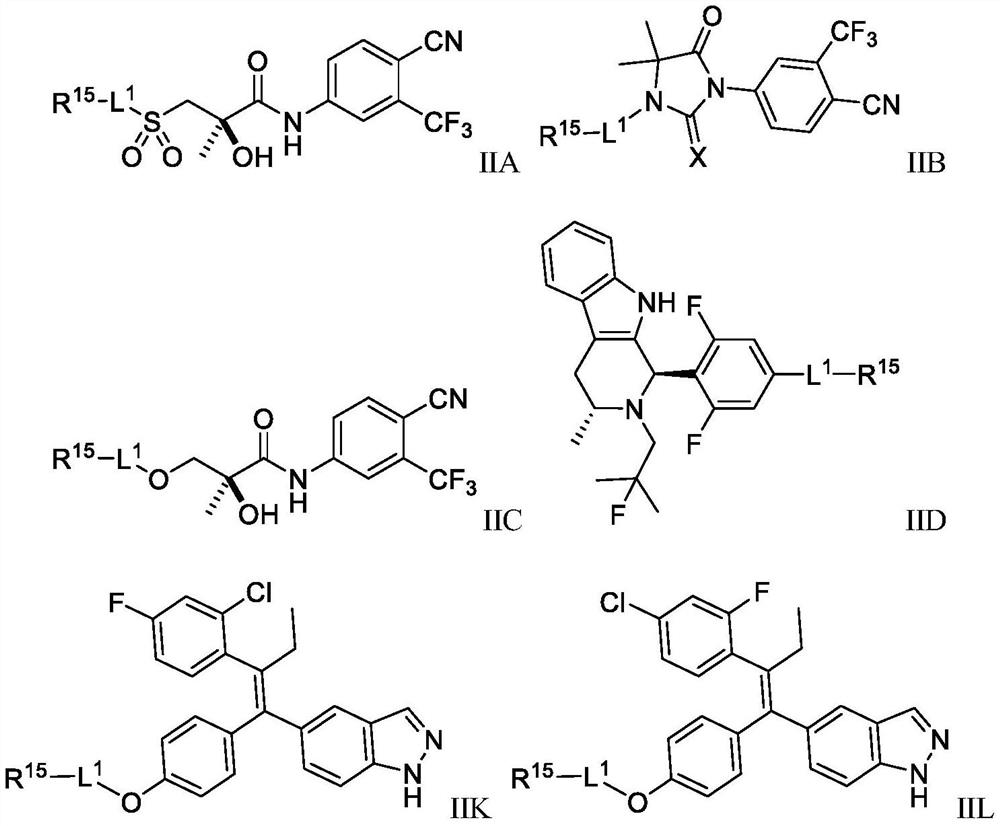
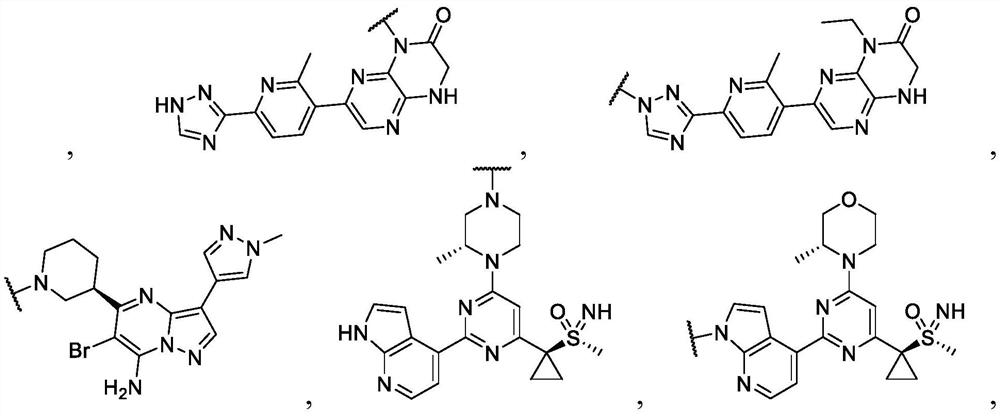
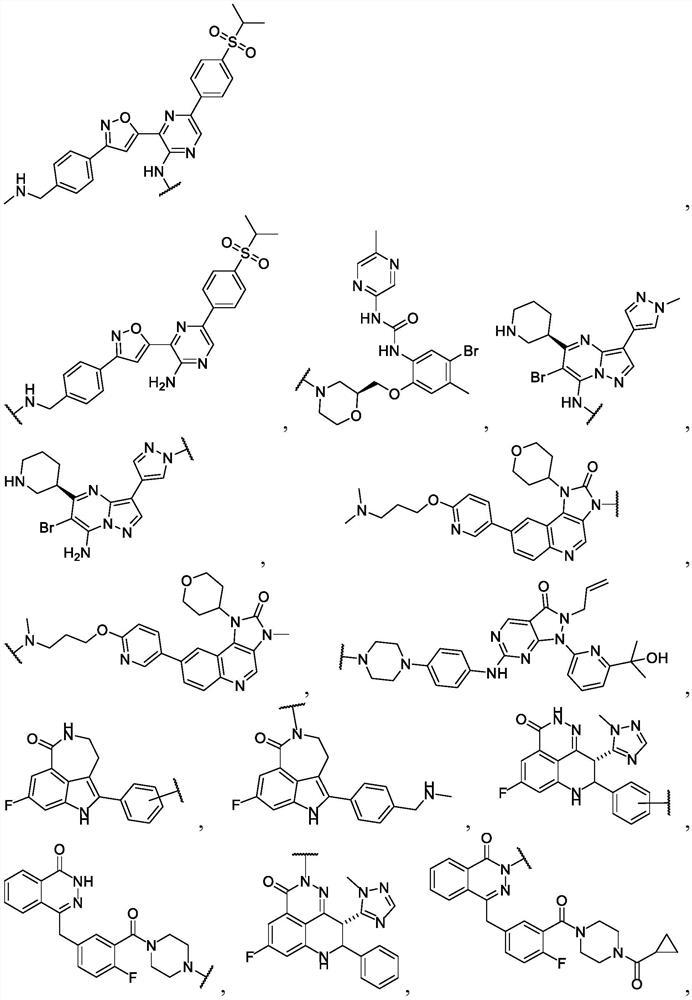
Anti-cancer nuclear hormone receptor-targeting compounds
A technology for compounds, mixtures, applied in, solvates, U.S. Provisional Application No. 2019, 62/, hydrates, isotope enrichment filed on November 13, 2019, capable of solving problems such as damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0572] The present disclosure is further illustrated by the following examples. The following examples are non-limiting and merely represent various aspects of the disclosure. Solid and dotted wedges within the structures disclosed herein illustrate relative stereochemistry, and absolute stereochemistry is only depicted when explicitly stated or described.
[0573] Compounds having the structure of any compound, formula, or any subformula described herein can be synthesized using standard synthetic techniques known to those of skill in the art. Compounds of the present disclosure can be synthesized using the general methods described or the general synthetic procedures illustrated in the Synthetic Examples.
[0574] When it is desired to obtain a specific enantiomer of a compound, this can be achieved from the corresponding enantiomeric mixture using conventional procedures applicable to the separation or resolution of enantiomers. Thus, for example, diastereomeric derivativ...
Embodiment S-1
[0624] Example S-1. Preparation of (S)-N-(4-cyano-3-(trifluoromethyl)phenyl)-3-(4-((8S,9R)-5-fluoro-9-( 1-methyl-1H-1,2,4-triazol-5-yl)-3-oxo-3,7,8,9-tetrahydro-2H-pyrido[4,3,2-de ]phthalazin-8-yl)phenoxy)-2-hydroxyl-2-methylpropionamide (compound 1.1a)
[0625]
[0626] Step 1: Preparation of (R)-3-bromo-N-(4-cyano-3-(trifluoromethyl)phenyl)-2-hydroxy-2-methylpropanamide
[0627] To a solution of (R)-3-bromo-2-hydroxy-2-methylpropionic acid (1.50 g, 3.79 mmol) in DMA (15 mL) was added thionyl chloride (1.50 g, 3.79 mmol) dropwise at 0 °C and The mixture was stirred at this temperature for 3 h. Then a solution of 4-amino-2-(trifluoromethyl)benzonitrile (0.84 g, 4.5 mmol, 1.2 equiv) in DMA (5 mL) was added to the mixture, and the mixture was stirred at room temperature for 16 h. The reaction was monitored by TLC. Upon completion, the mixture was concentrated under reduced pressure. The combined organic layers were washed with saturated NaHCO 3 solution (50mL), water (5...
Embodiment S-2
[0630] Example S-2. Preparation of (S)-N-(4-cyano-3-(trifluoromethyl)phenyl)-3-(4-((8R,9S)-5-fluoro-9-( 1-methyl-1H-1,2,4-triazol-5-yl)-3-oxo-3,7,8,9-tetrahydro-2H-pyrido[4,3,2-de ]phthalazin-8-yl)phenoxy)-2-hydroxyl-2-methylpropionamide (compound 1.1b)
[0631]
[0632] To (8R,9S)-5-fluoro-8-(4-hydroxyphenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8 at 0°C , To a stirred solution of 9-dihydro-pyrido[4,3,2-de]phthalazin-3(7H)-one (100 mg, 0.26 mmol) in DMF (5 mL) was added sodium cyanide (60% suspension in mineral oil; 20.8 mg, 0.52 mmol), followed by (R)-3-bromo-N-(4-cyano-3-(trifluoromethyl)phenyl)-2-hydroxy-2-methyl Propionamide (0.44 g, 0.31 mmol, 2 equiv) and the resulting mixture was heated at 90 °C for 16 h. The reaction was monitored by TLC and LCMS. Upon completion, the reaction was quenched with ice-cold water and extracted with EtOAc (50 mL), the organic layer was washed with water (50 mL), brine (50 mL), washed with Na 2 SO 4 Dried, filtered and concentrated u...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap