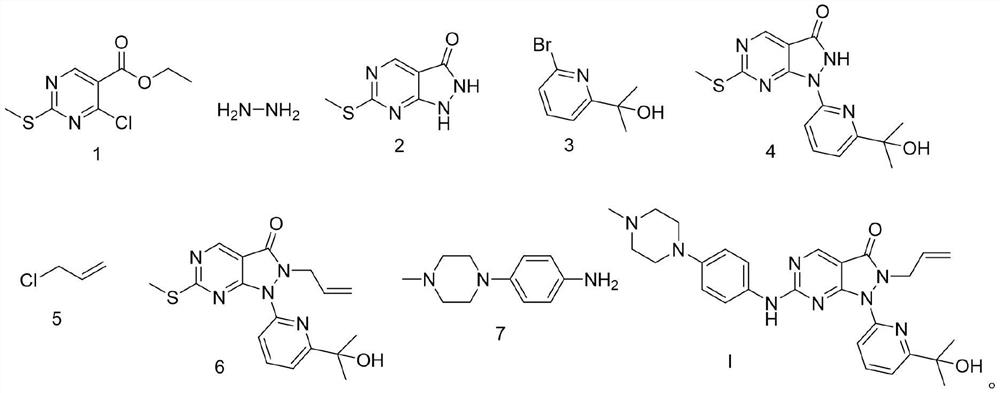
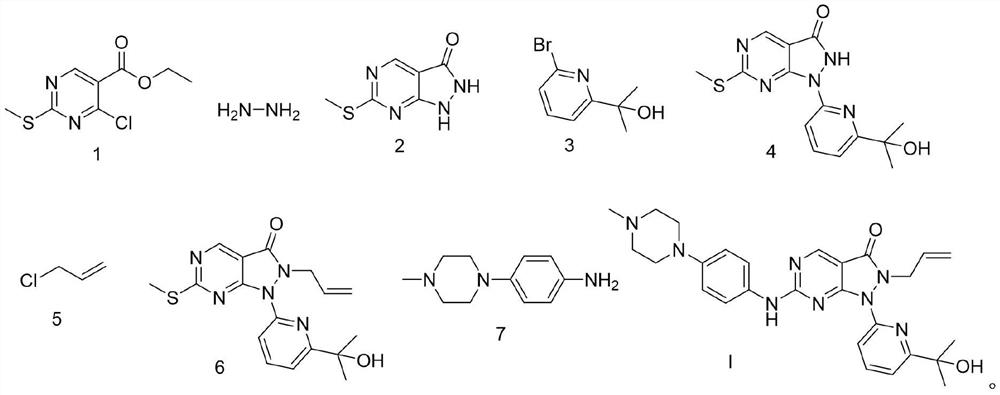
Novel chemical synthesis method of Wee1 protein kinase inhibitor adavisertib
A chemical synthesis and new method technology, applied in the field of biomedicine, can solve the problems that need to be improved, and achieve the effects of high overall reaction yield, simple post-reaction treatment operation, and reduced reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 7
[0063] Embodiment 7 is a comparative example. In this embodiment 7, the inventor adjusted the compound shown in formula 2, K 2 CO 3 , and the reaction molar ratio of compound shown in formula 3 is 1:1.5:1.3, and in its technical effect, the product yield obtained is lower than compound shown in formula 2, K 2 CO 3 , The product yield when the molar ratio of the compound shown in formula 3 is 1:(1.0~1.2):(1.0~1.1).
[0064] At room temperature, compound (3.50g, 19.21mmol) shown in formula 2 and K 2 CO 3 (3.98g, 28.80mmol) was added to stirring DMF (25mL) and mixed, continued to stir, added the compound shown in formula 3 (5.40g, 24.99mmol), heated to 60°C and stirred for 2h. After the reaction was complete, the reaction solution was poured into Pour into water (25mL), collect the precipitated solid by filtration, use a mixed solvent (40mL) composed of petroleum ether / ethyl acetate with a volume ratio of 2:1 to stir and beat for purification for 30min, filter the solid, and ...
Embodiment 11
[0073] Embodiment 11 is a comparative example. In this embodiment 11, the inventor adjusted the compound shown in formula 4, Cs 2 CO 3 , The reaction molar ratio of the compound shown in Formula 5 is 1:1.8:1.5, and the reaction time is extended from 5 hours to 6 hours. In the technical effect of present embodiment 11, the product yield obtained is lower than the compound shown in formula 4, Cs 2 CO 3 , the product yield when the molar ratio of the compound shown in formula 5 is 1:(1.1~1.5):(1.05~1.2), and the impurities in the compound shown in the obtained formula 6 increase.
[0074] At room temperature, the compound shown in formula 4 (4.00g, 12.60mmol) and Cs 2 CO 3 (7.39g, 22.68mmol) was added into stirring DMF (60mL), and then the compound represented by formula 5 (1.45g, 18.95mmol) was added, and the mixture was heated to 105°C and stirred for 6h. After the reaction was completed, the reaction solution was poured into water (60 mL). After the precipitated solid wa...
Embodiment 12
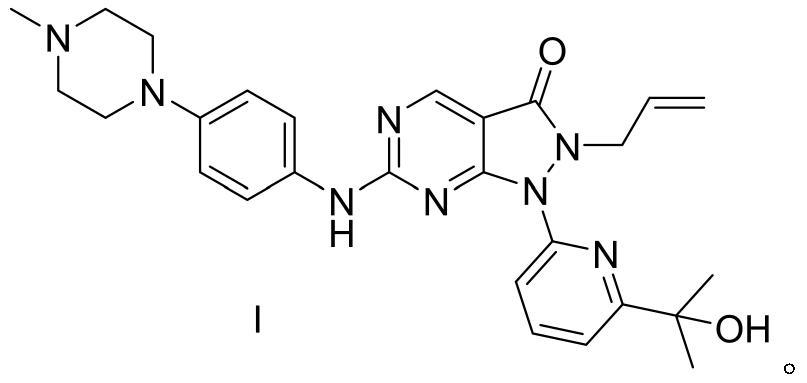
[0075] Synthesis of compound Adavosertib shown in embodiment 12 formula I
[0076] At room temperature, add the compound represented by formula 6 (35.7 g, 0.1 mol) and m-chloroperoxybenzoic acid (mCPBA) (19.0 g, 0.11 mol) into toluene (300 mL), and keep stirring at room temperature for 1 h to obtain a reaction solution , and then add N,N-diisopropylethylamine (DIPEA) (64.6g, 0.5mol) and the compound shown in formula 7 (21.0g, 0.11mol) to the reaction solution, and keep the reaction mixture at room temperature and stir for 18h reaction. After the reaction is complete, add saturated NaHCO 3 solution (300ml), the mixture was extracted with ethyl acetate (2×150ml), the organic layer was washed with saturated brine (300ml), dried over sodium sulfate, filtered, and the filtrate was concentrated with dichloromethane / A mixed solvent of methanol was purified by silica gel column chromatography to obtain the compound of formula I, Adavosertib, with a solid yield of 39.7 g, a yield of...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap