S100A12 as blood biomarker for non-invasive diagnosis of endometriosis
A technology of S100A12, endometriosis, applied in the direction of disease diagnosis, biological testing, biomaterial analysis, etc., can solve the problem of limited diagnostic utility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0080] In a first aspect, the present invention relates to a method of assessing whether a patient suffers from or is at risk of developing endometriosis, comprising
[0081] a) determining the amount of said S100A12 in a sample from said patient, and
[0082] b) Comparing the determined quantity with a reference.
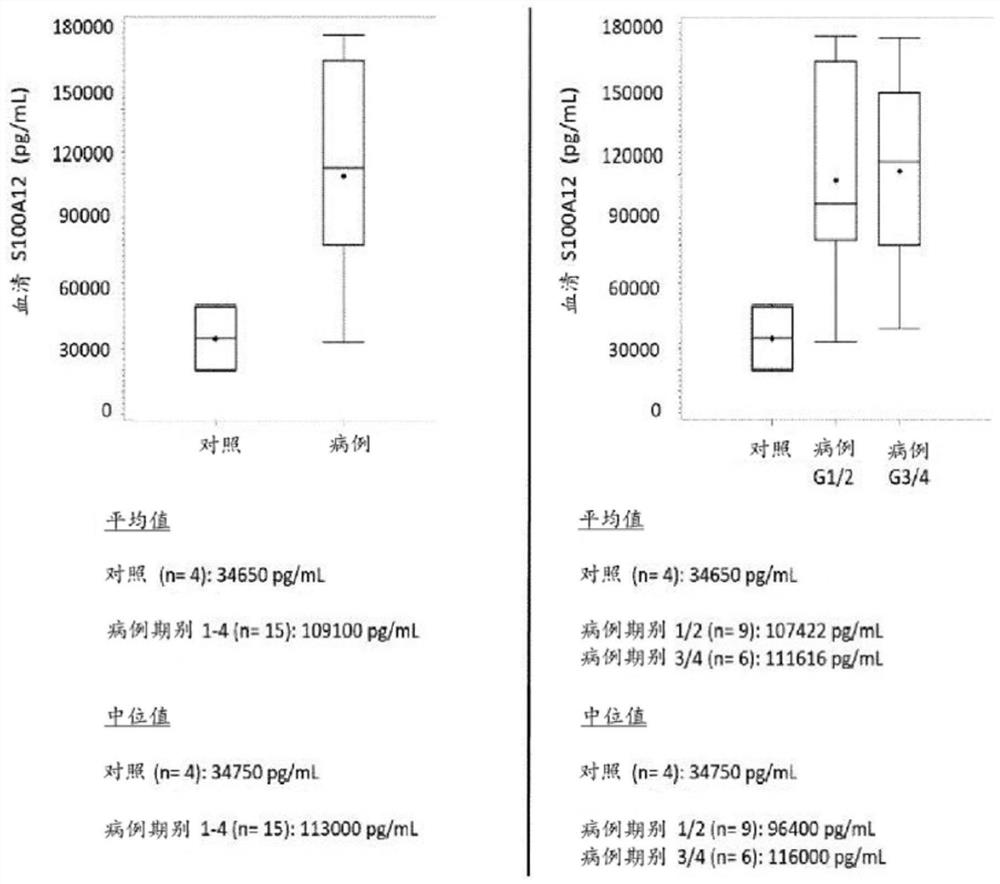
[0083] In an embodiment, an elevated amount of S100A12 in a patient sample indicates that the patient has or is at risk of developing endometriosis. In particular, if the amount of S100A12 in the patient sample is higher than the reference or the amount of S100A12 in the reference sample, the amount of S100A12 in the patient sample indicates that the patient has endometriosis or is at risk of developing endometriosis. In particular, in patients assessed as having endometriosis or at risk of developing endometriosis compared to the same fluid sample from individuals without endometriosis or at risk of developing endometriosis Higher amounts of S100A12 were detecte...
example 1
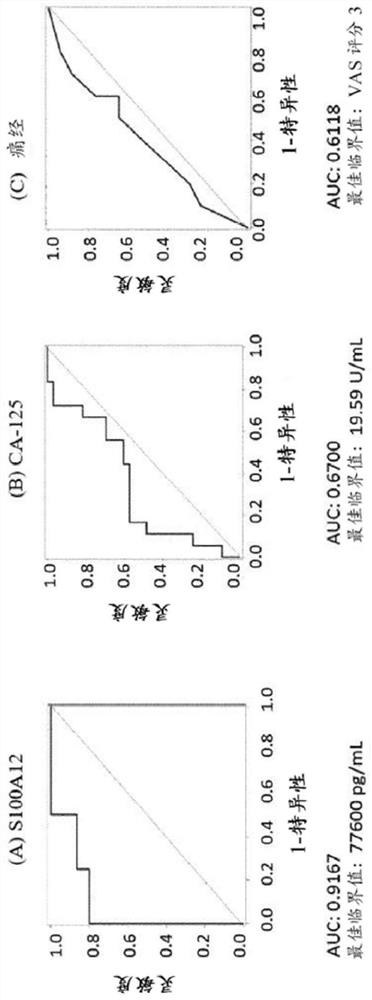
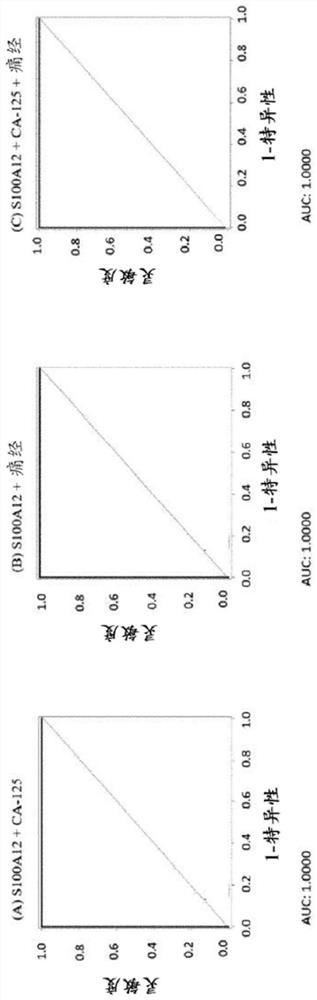
[0175] Example 1: Diagnostic performance of biomarker S100A12 and biomarker combinations in women with endometriosis and controls
[0176] For the measurements, a total of 21 serum and 31 plasma samples from human females were analyzed. Analyte concentrations were determined by ELISA (Enzyme-Linked Immunosorbent Assay). The case group included patients with laparoscopy-diagnosed pelvic endometriosis (rASRM stages I-IV), and the control group included healthy women without endometriosis.
[0177] The concentration of S100A12 in human serum was determined using the Human S100A12 / EN-RAGE ELISA Kit Ver. 2 from CircuLex / MBL (distributed by Biozol Eching, Germany; catalog number: CY-8058V2). The kit uses quantitative sandwich ELISA technology. Microtiter plates were pre-coated with a monoclonal antibody specific for human S100A12. Samples were measured at 200-fold dilutions. After bringing all reagents to room temperature, add 100 µL of each sample and standard. Sample single m...
example 2
[0193] Example 2: Diagnostic performance of biomarker S100A12 and biomarker combinations in samples from a multicenter study in women with endometriosis and controls.
[0194] The case groups included patients diagnosed with peritoneal endometriosis, deep infiltrating endometriosis, and endometriosis. Endometriosis (rASRM stages I-IV) was diagnosed by laparoscopic visualization and subsequent histological confirmation, and controls included healthy women without endometriosis. Inclusion criteria for the case group were presence of pelvic pain / infertility and age between 18-45 years. The exclusion criteria for the case group were pregnancy / lactation, malignancy, recurrent endometriosis, and laparoscopy / laparotomy ≤6 months.
[0195] S100A12 was measured using the pre-commercial ECLIA assay for S100A12, a cobas Sandwich immunoassay developed on the ECLIA platform (ECLIA assay from Roche Diagnostics, Germany). The assay includes biotinylated and ruthenated monoclonal antibodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com