Treatment of insulin resistance associated with prolonged physical inactivity
A technique for insulin resistance, physical activity, applied in the field of biomarkers, to address issues such as poor quality of life outcomes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
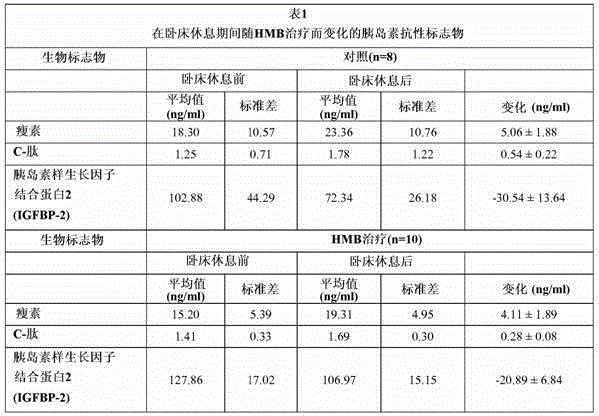
[0126] Example 1: Effect of HMB intervention on circulating levels of IGFBP-2 in subjects who have undergone 10 days of bed rest.
[0127] IGF-binding proteins prolong the half-life of IGF and alter the interaction of IGF with their cell surface receptors. Reduction of circulating IGFBP-2 is associated with increased insulin resistance in human subjects.
[0128] As shown in Table 1, there was a mean decrease of -30.54 ng / ml or -24.56% decrease in circulating levels of IGFBP-2 in 8 control subjects after 10 days of bed rest. In contrast, the average decrease in IGFBP-2 levels in blood from 10 subjects treated with HMB was much less. As shown in Table 1, there was a mean decrease of -20.89 ng / ml or -15.63% decrease in blood levels of IGFBP-2 in these HMB-treated subjects. These results indicate that HMB attenuates or attenuates the decrease in blood levels of IGFBP-2 that occurs during prolonged bed rest in control subjects.
Embodiment 2
[0129] Example 2: Effect of HMB intervention on circulating levels of C-peptide in subjects who have undergone 10 days of bed rest.
[0130] The beta cells of the pancreas produce C-peptide (Swiss-Prot accession number: P01308) as part of the proinsulin molecule (insulin precursor). Increases in circulating C-peptide are associated with insulin resistance in human subjects.
[0131] As shown in Table 1, there was a mean increase in circulating levels of C-peptide of 0.54 ng / ml or a mean increase of 43.0% in 8 control subjects after 10 days of bed rest. In contrast, the average increase in C-peptide levels in blood from the 10 HMB-treated subjects was much greater. As shown in Table 1, there was a mean increase of 0.28 ng / ml or a 29.59% increase in blood levels of C-peptide in these HMB-treated subjects after 10 days of bed rest. These results indicate that HMB attenuates or attenuates the increase in blood levels of C-peptide that occurs during prolonged bed rest in untrea...
Embodiment 3
[0132] Example 3: Effect of HMB intervention on circulating levels of leptin in subjects who have undergone 10 days of bed rest.
[0133] Leptin is a 16-kDa adipocyte-derived hormone that circulates in serum in both free and bound forms (MantzorosCS, Ann Intern Med 1999;130:671-680). Studies have demonstrated that insulin resistance is associated with elevated plasma leptin levels independent of body fat mass (Segal KR, et al., Diabetes 1996;45:988-991.).
[0134]As shown in Table 1, there was a mean increase in circulating levels of leptin of 5.06 ng / ml in 8 control subjects after 10 days of bed rest. In contrast, there was a lower mean increase in leptin circulating levels of 4.11 ng / ml in the 10 HMB-treated subjects after 10 days of bed rest. These values were significantly lower than control values. These results indicate that HMB attenuates or attenuates the increase in blood levels of leptin that occurs during prolonged bed rest in untreated control subjects. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com