Anti-serine protease inhibitor KAZAL (SPIK) antibodies, immunoconjugates and methods of use
An antibody and amino acid technology, applied in the direction of anti-peptide protease inhibitor immunoglobulin, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problem of high cost imaging technology, low sensitivity and small detection Tumors are less accurate and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
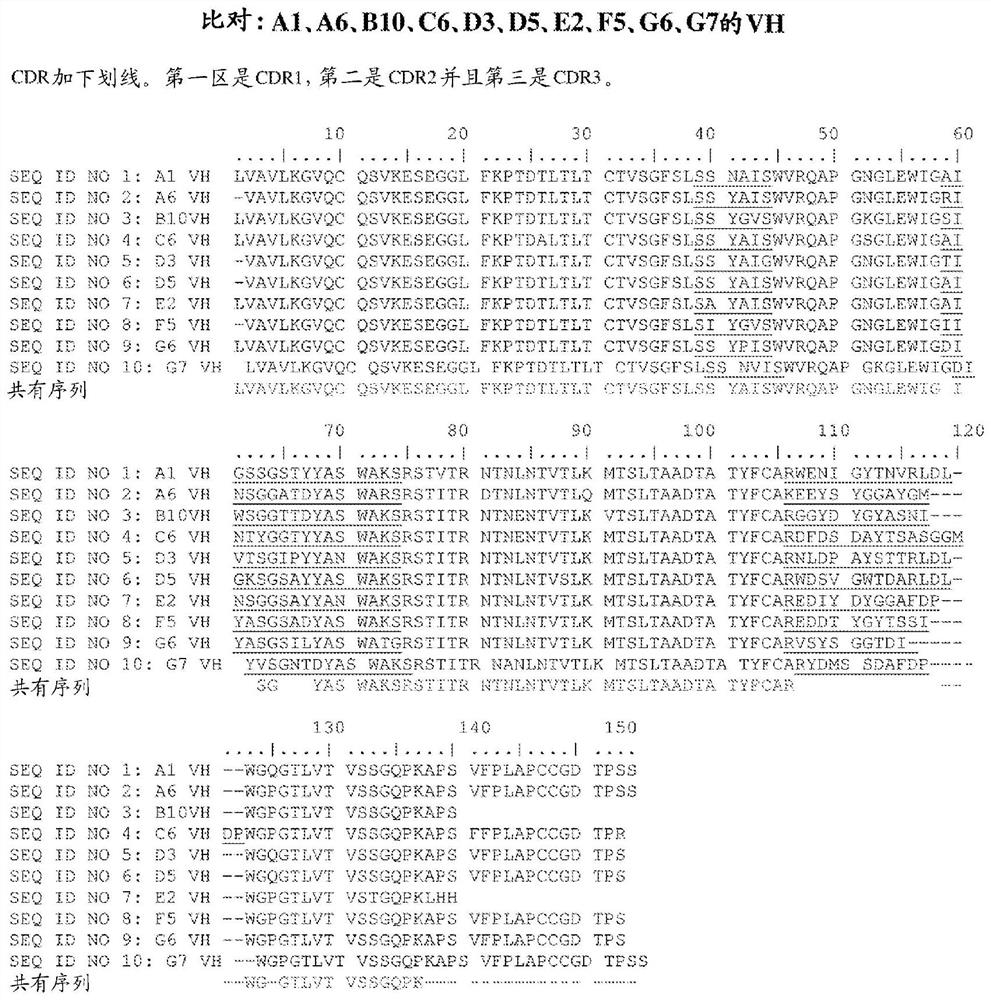
[0101] The present invention is based at least in part on the discovery that certain disorders are characterized by the expression of a unique form or serine protease inhibitor Kazal (SPIK). A prominent example is liver cancer, which includes but is not limited to hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC). More specifically, the inventors have discovered that certain cancers, such as liver cancer, express a form of SPIK that includes an additional 23 amino acids at the N-terminus of the secreted SPIK polypeptide. This 23 amino acid stretch (SEQ ID NO: 81 ) is not found in SPIK polypeptides secreted by normal cells such as pancreatic cells. This is consistent with a previous report that the first 9 amino acids of this 23 amino acid stretch can be present in unprocessed SPIK secreted by hepatoma cell lines. Lu et al., Immunology 2011;134(4):398-408. The longer form of SPIK may be referred to as AS-SPIK or aberrantly secreted SPIK. AS-SPIK produc...
Embodiment 1
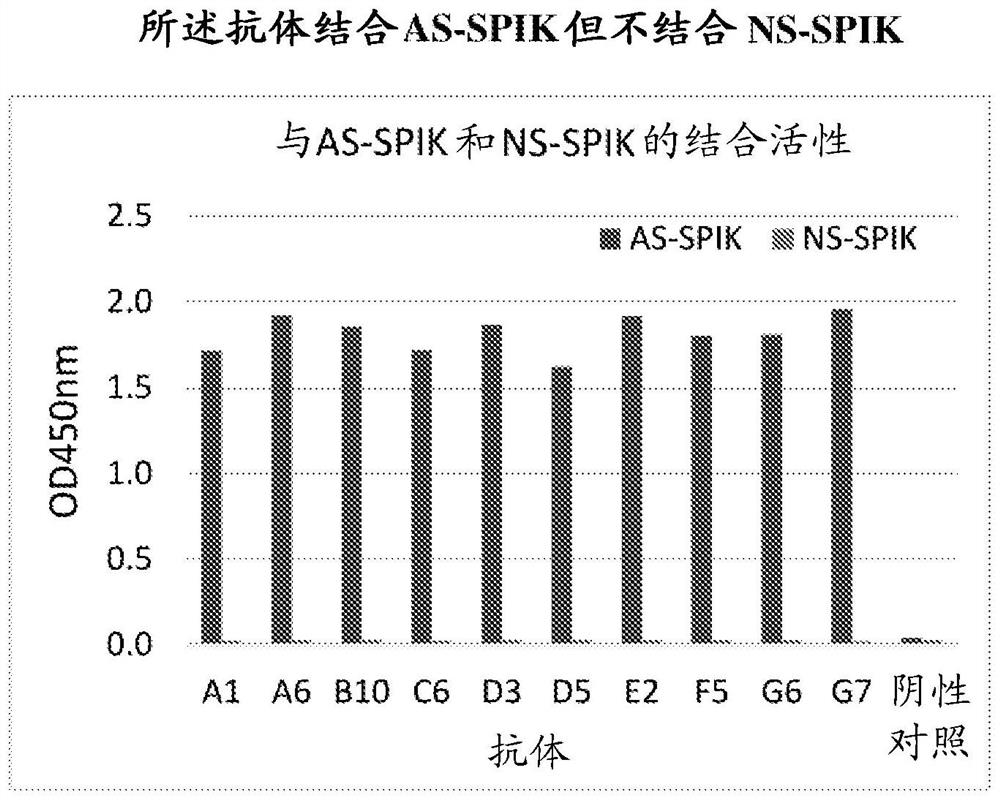
[0243] Example 1: Preparation of Rabbit Monoclonal Antibody Specific to AS-SPIK
[0244] The ten monoclonal antibodies described here were raised from rabbits. Briefly, rabbits were immunized with different subsets of recombinant AS-SPIK containing 23 additional amino acids (SEQ ID NO:81). Blood was tested by ELISA after three or four infusions. Briefly, blood from immunized rabbits was reacted with plates coated with recombinant AS-SPIK. After washing, the color was developed by incubating the plate with an anti-rabbit antibody labeled with HRP (horseradish peroxidase), and the optical density was measured after reaction with the substrate TMB. Rabbits that tested positive (antibody production) were sacrificed. Monoclonal antibodies were then established and immobilized with cells from S2-3 (a cell line expressing large amounts of AS-SPIK through the integrated entire SPIK gene, Lu et al. Tumor-associated protein SPIK / TATI suppresses serine protease dependent cell apoptosi...
Embodiment 2
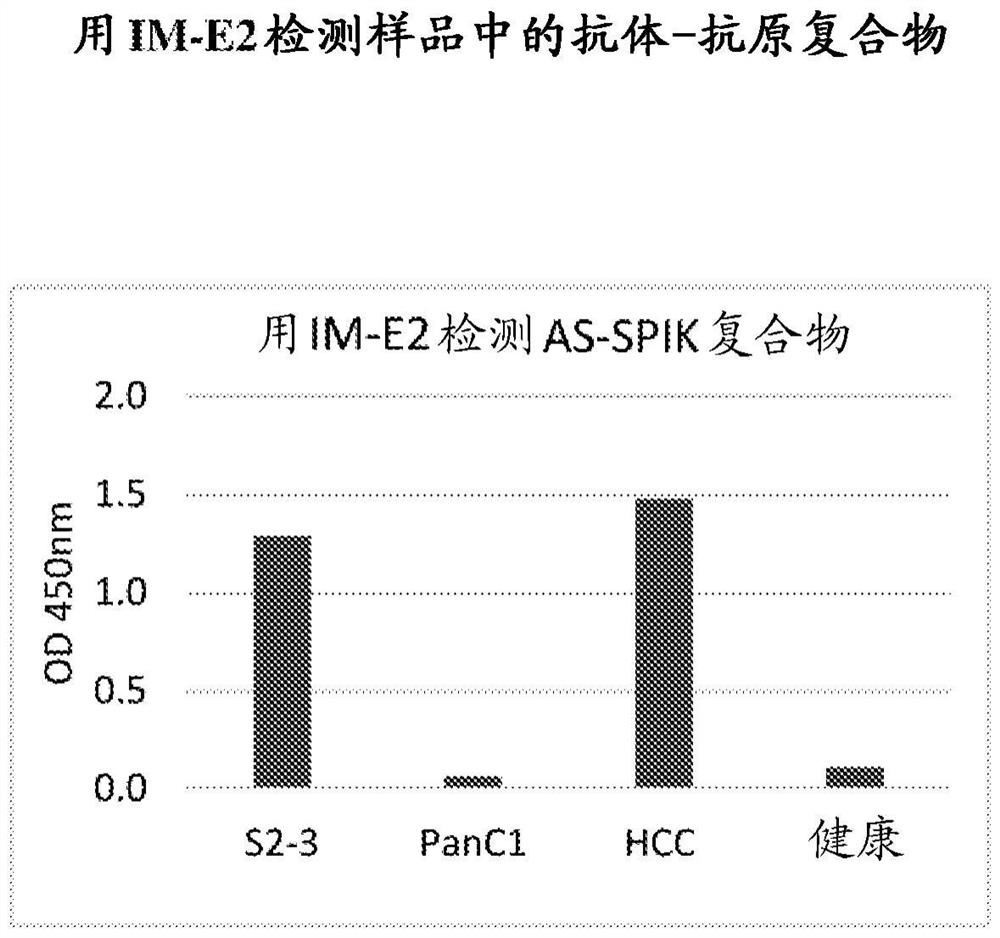
[0245] Example 2: Detection of antibody-antigen complexes in samples with IM-E2
[0246] Measured by sandwich ELISA by antibodies IM-A1, IM-A6, IM-B10, IM-C6, IM-D3, IM-D5, IM-E2, IM-F5, IM-G6 and IM-G7 in combination with AS-SPIK Complexes formed in medium of S2-3, medium of PanC1, sera of HCC patients and healthy persons. Briefly, immune complexes with antibodies to AS-SPIK in different samples were formed by incubating the antibodies with the samples at 37°C for 0.5 hours. The mixture was then reacted with a 96-well plate immobilized with polyclonal anti-SPIK antibody at 37°C for 1 hour. Plates were then incubated with HRP-labeled anti-rabbit antibody for 45 minutes at 37°C. After reaction with the substrate TMB, the amount of antibody-antigen complex was determined by optical density. figure 2 Test results from IM-E2 are shown. Similar results were obtained with other antibodies. The results clearly showed that IM-E2 was able to form antibody-antigen complexes with A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com