Cariprazine pharmaceutical salt and crystal form, preparation method and application thereof
A technology of cariprazine and medicinal salt, which is applied in the field of cariprazine medicinal salt and its crystal form, preparation and application, which can solve the problems of poor medication compliance of patients, and achieve good market prospect and stable crystal form Good performance and good storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
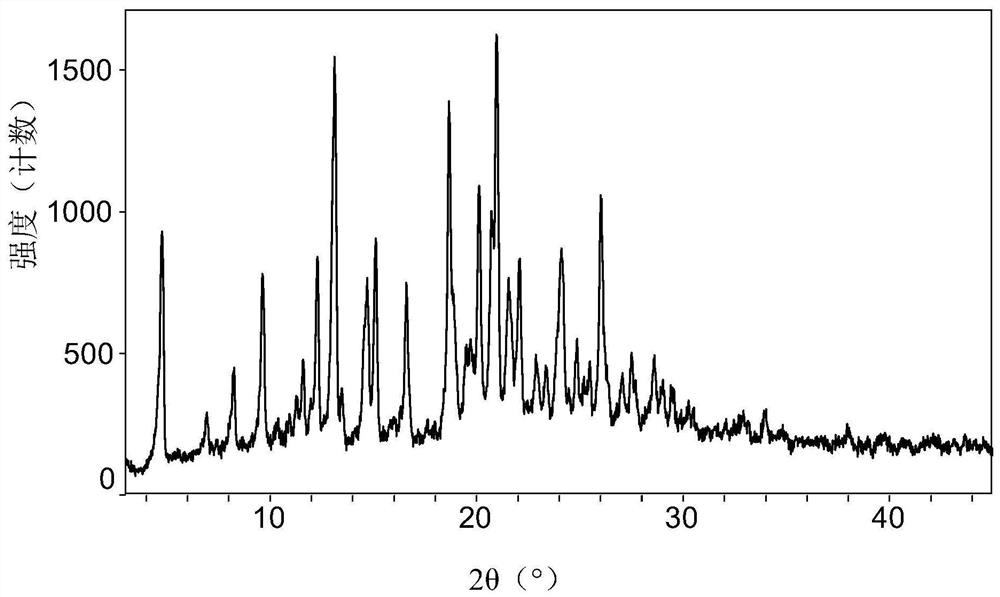
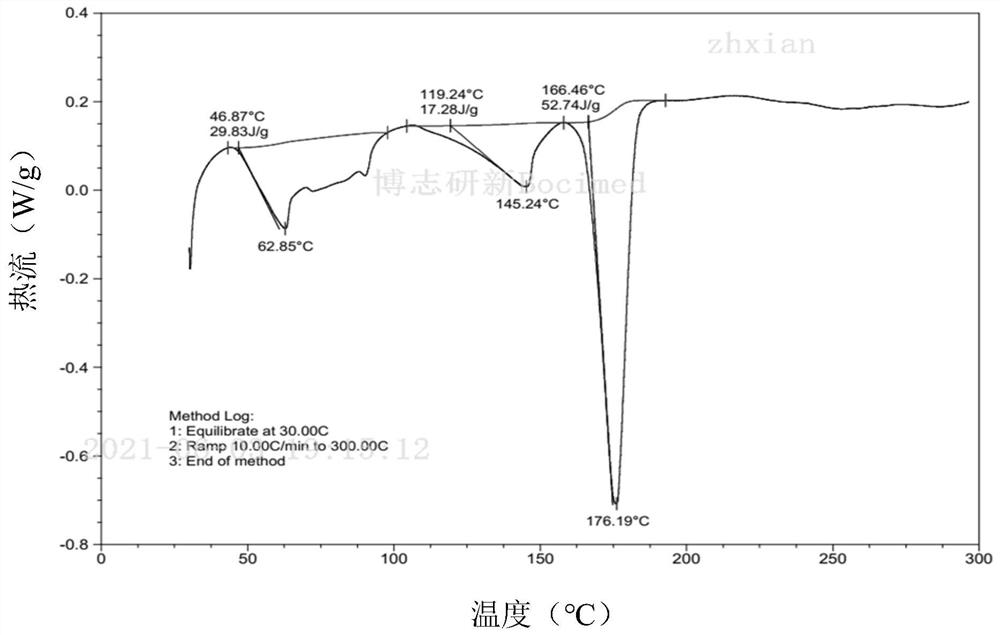
[0142] Embodiment 1: the preparation of cariprazine pamoate
[0143]Dissolve 4000mg (9.36mmol) cariprazine in 200mL (5.4mg / mL) phosphoric acid solution to obtain solution A; dissolve 3634mg (9.36mmol) pamoic acid in 100mL (7.5mg / mL) sodium hydroxide solution In, solution B was obtained. Under stirring, 100 mL of solution B was added to 200 mL of solution A within 30 minutes, the product was separated by filtration and rinsed with water, and vacuum-dried at 40° C. for 12 hours to obtain 5840 mg of a light yellow solid with a yield of 76% (calculated as free base).
[0144] The structure and molar ratio of the cariprazine pamoate described in the present invention are confirmed by proton nuclear magnetic resonance spectrum.
[0145] 1 H-NMR (400MHz, DMSO-d6): δ8.38(s,2H), 8.16(d,2H), 8.80(d,2H), 7.39-7.13(m,7H), 5.86(d,1H), 4.76(s,2H), 3.40-3.32(m,3H), 3.22-3.18(m,4H), 2.75(s,6H), 1.76(t,4H), 1.63-1.57(m,2H), 1.25- 1.16(m,3H), 1.04-0.96(m,2H).
[0146] NMR results showed th...
Embodiment 2
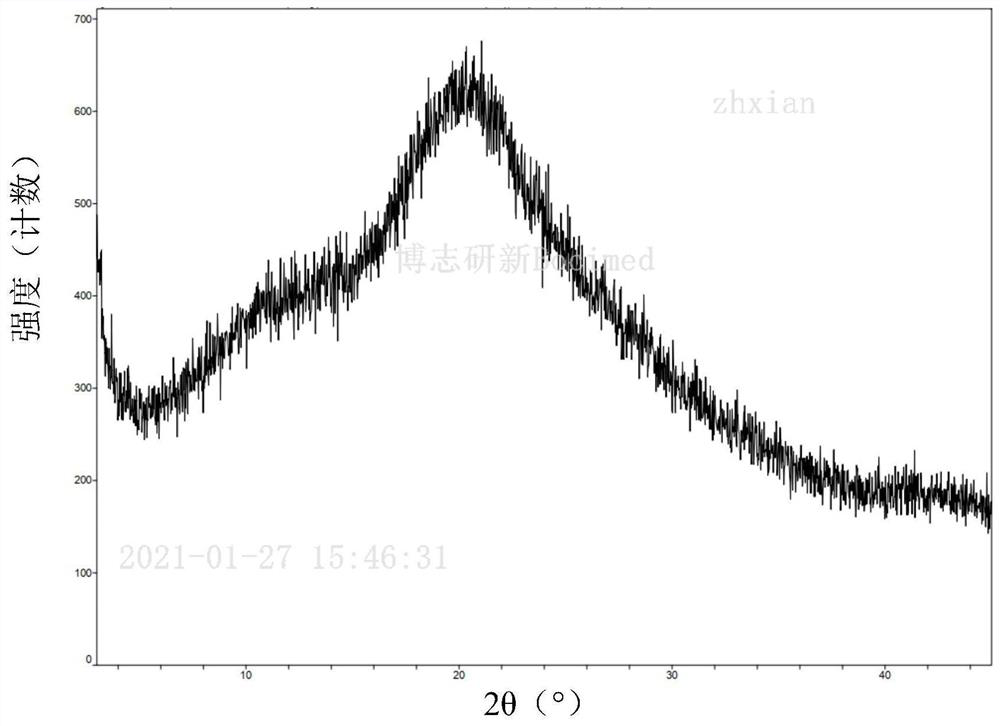
[0148] Example 2: Preparation of Cariprazine Pamoate Amorphous Form
[0149] Under the condition of 60°C, 20g (46.8mmol) cariprazine and 18.17g (46.8mmol) pamoic acid were dissolved in 170mL THF:MeOH (2:1) mixed solvent, filtered, concentrated under reduced pressure to remove the solvent, and then Add 300mL of methanol, dissolve at 60°C, concentrate under reduced pressure to remove the solvent, and dry under vacuum at 40°C for 12 hours to obtain 36.6g of amorphous cariprazine pamoate.
Embodiment 3
[0150] Embodiment 3: Preparation of cariprazine hemipamoate
[0151] Dissolve 200mg (0.468mmol) cariprazine in 10mL (5.4mg / mL) phosphoric acid solution to obtain solution A; dissolve 90.85mg (0.234mmol) pamoic acid in 2.5mL (7.5mg / mL) hydrogenation Sodium solution to obtain solution B. With stirring, 2.5 mL of solution B was added to 10 mL of solution A within 30 minutes, the product was separated by filtration and washed with water, and dried in vacuum at 40° C. for 12 hours to obtain 204 mg of light yellow solid with a yield of 70%.
[0152] The structure and molar ratio of the cariprazine pamoate described in the present invention are confirmed by proton nuclear magnetic resonance spectrum.
[0153] 1 H-NMR (400MHz, DMSO-d6): δ8.25(s,1H), 8.18(d,1H), 7.69(d,1H), 7.38-7.31(m,2H), 7.22-7.14(m,2H ), 7.05(t,1H), 5.86(d,1H), 4.71(s,1H), 3.39-3.22(m,5H), 2.75(s,6H), 1.75(t,4H), 1.59-1.54( m, 2H), 1.25-1.15 (m, 3H), 1.04-0.95 (m, 2H).
[0154] NMR results showed that caripraz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com