Methods of improving spinal fusion with abavatide
A technology of abaloparatide and spine, applied in the field of enhancing spinal fusion, promoting rehabilitation after spinal surgery, and promoting bone formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
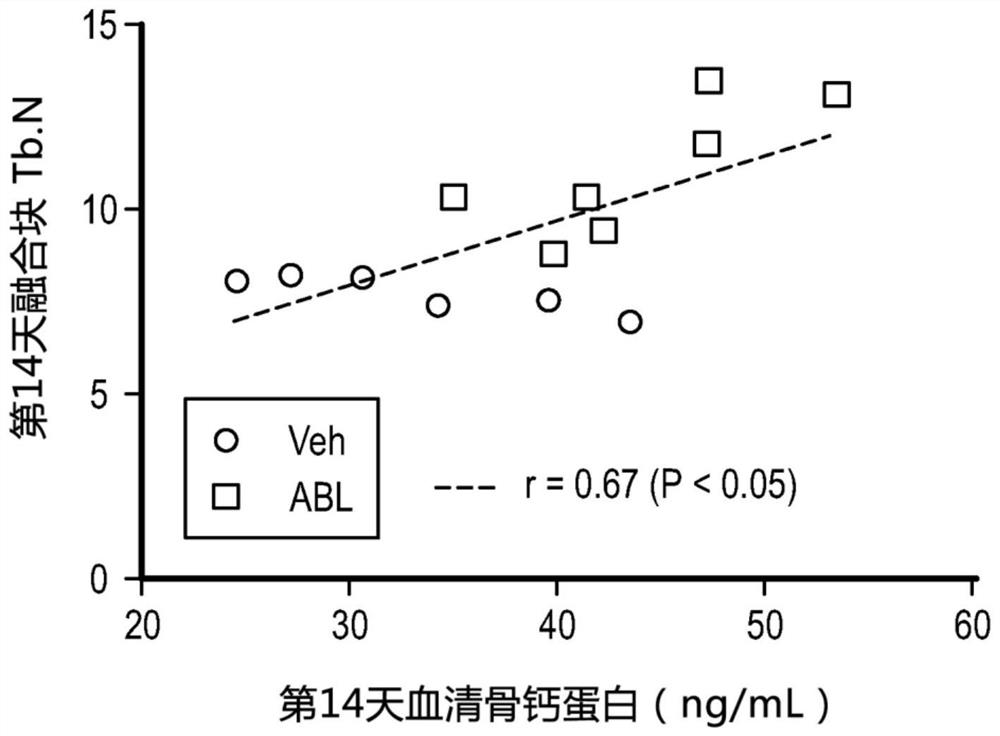
[0058] The data presented in Example 1 contained excellent inter-observer and inter-modality concordance in fusion assessments. It is also noteworthy that the positive effect was achieved with a relatively modest dose of abaloparatide, which represents a significant improvement in its approved clinical dose compared to the dose of teripatide used in most rat spinal fusion studies. Substantially lower multiple. When dosed as low as 5 μg / kg / d, abaloparatide increases spinal BMD and promotes fracture bridging and callus strength in rats.
Embodiment 2
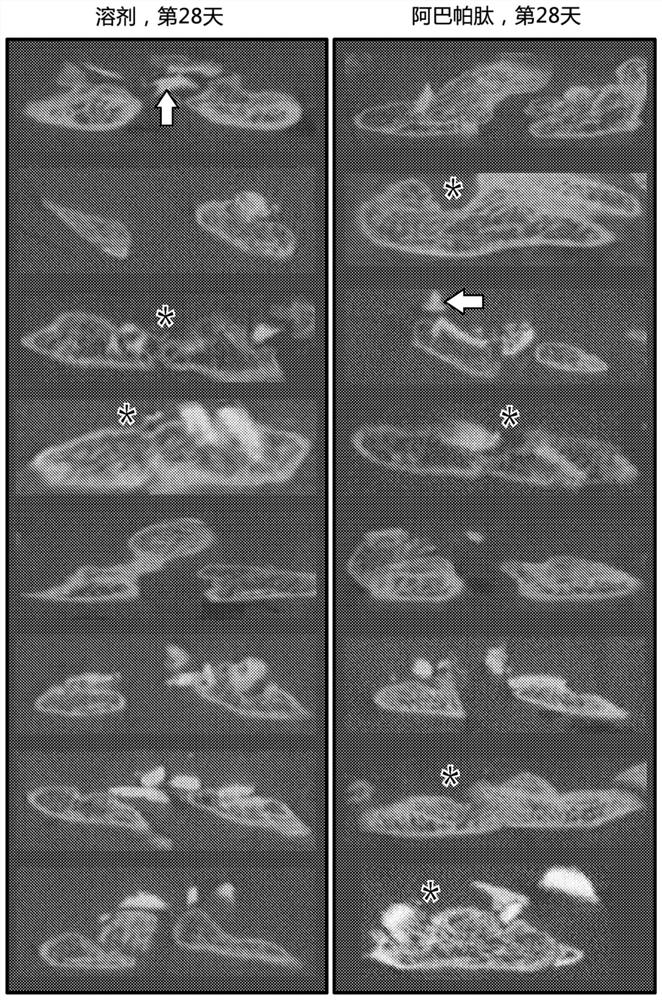
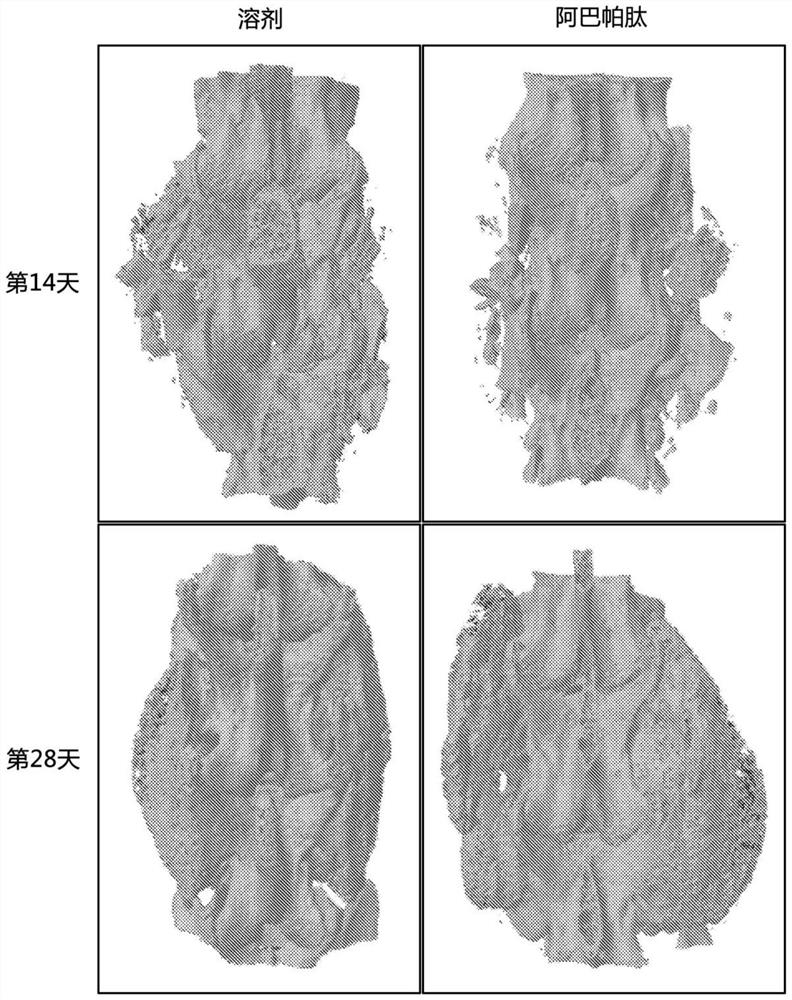
[0059] The data presented in Example 2 show that acceptable rates of spinal fusion were achieved in all Spinal Arthrodesis Model rabbits, with a success rate of 45% (5 / 11) in the control group. Across all metrics studied, better outcomes for spinal fusion surgery were observed in the group of rabbits given abaloparatide. A statistically significantly higher number of spines were determined (by MAF) to be acceptably fused. Larger and stronger fusion masses were also identified in abaloparatide-treated rabbits. Abaloparatide successfully improves the outcome of spinal fusion surgery in a large animal model (rabbit).
[0060] The following examples are provided to better illustrate the claimed method and should not be construed as limiting the scope of the method provided by the present invention. Where specific materials are mentioned, they are for purposes of illustration only and are not intended to be limiting of the invention. Those skilled in the art may develop equivale...
Embodiment
[0062] Example 1: Abaloparatide Enhances Spinal Fusion in a Rat Posterolateral Fusion Model
[0063]Data presented herein demonstrate that abaloparatide enhances spinal fusion in a rat posterolateral model (PLF) and a rabbit spinal arthrodesis model. Due to the positive effect of PTHR1 activation on spinal fusion observed in several studies and the potentially favorable overall pharmacodynamic profile of ABL with increased bone formation and minimal bone resorption, the efficacy of ABL in spinal fusion was investigated. ABL use had positive early effects on fusion mass structure in rats undergoing non-instrumented PLF.
[0064] The aim of this study was to evaluate the in vivo performance of daily injections of ABL20 (20 μg / kg) compared to saline controls in an established rat model of posterolateral fusion. Thirty-two 10-week-old male rats underwent posterolateral spinal surgery. A dorsal midline skin incision was made from L1 to the sacrum, followed by dissection of fascia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com