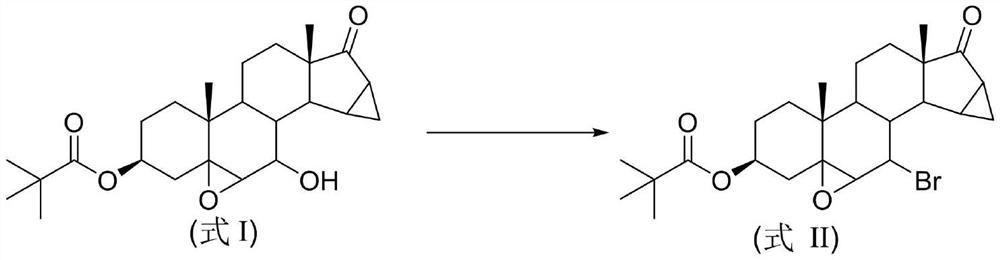
Synthesis method of drospirenone key intermediate bromide
A synthesis method and technology of intermediates, applied in steroids, organic chemistry and other directions, can solve the problems of poor selectivity, large amount of phosphorus-containing waste liquid, large amount of organic alkali, etc., and achieve convenient post-processing, high atom economy, The effect of reducing usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Add 2.08g (5mmol) of 3-pivaloyloxy-5β,6β-epoxy-7β-hydroxyl-15β,16β-dimethylene-pregna-17-one, 0.13g (1mmol) aluminum chloride, 0.65g (7.5mmol) lithium bromide and 15g silica gel, then add stainless steel balls with a diameter of 8mm and place them in the ball mill, set the operating frequency of the ball mill to 12Hz, and stop the mechanical grinding after 0.5h. The entire reaction mixture was transferred from the grinding tank to a beaker, added 15 mL of dichloromethane to soak for 1 hour, filtered, and the filtrate was concentrated to dryness, and then recrystallized by adding 33 mL of a mixed solvent of ethanol and water (volume ratio: 10:1) to obtain 2.03 g of white solid 3-pivaloyloxy-5β,6β-epoxy-7-bromo-15β,16β-dimethylene-pregna-17-one, yield 85%.
[0027] After testing, the specific characteristics of the product are as follows:
[0028] Melting point: 198-201℃, 1 H NMR (600MHz, CDCl 3 )δ4.86-4.75(m,1H),4.74-4.71(m,1H),3.43(d,1H,J=2.4Hz),2.15-2.03(m...
Embodiment 2
[0030] According to the method and steps of Example 1, the only difference is that in step (1), the molar ratio of raw material and Lewis acid is adjusted to 1:0.5, and the yield is 88%.
Embodiment 3
[0032] According to the method and steps of Example 1, the only difference is that in step (1), the molar ratio of the raw material and the brominating reagent is adjusted to 1:1, and the yield is 74%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com