Lactate dehydrogenase inhibitor polypeptides for treatment of cancer
A lactate dehydrogenase and cyclic polypeptide technology, which is applied in the field of polypeptides that regulate the activity of natural tetramer lactate dehydrogenase, can solve the problem of low clinical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
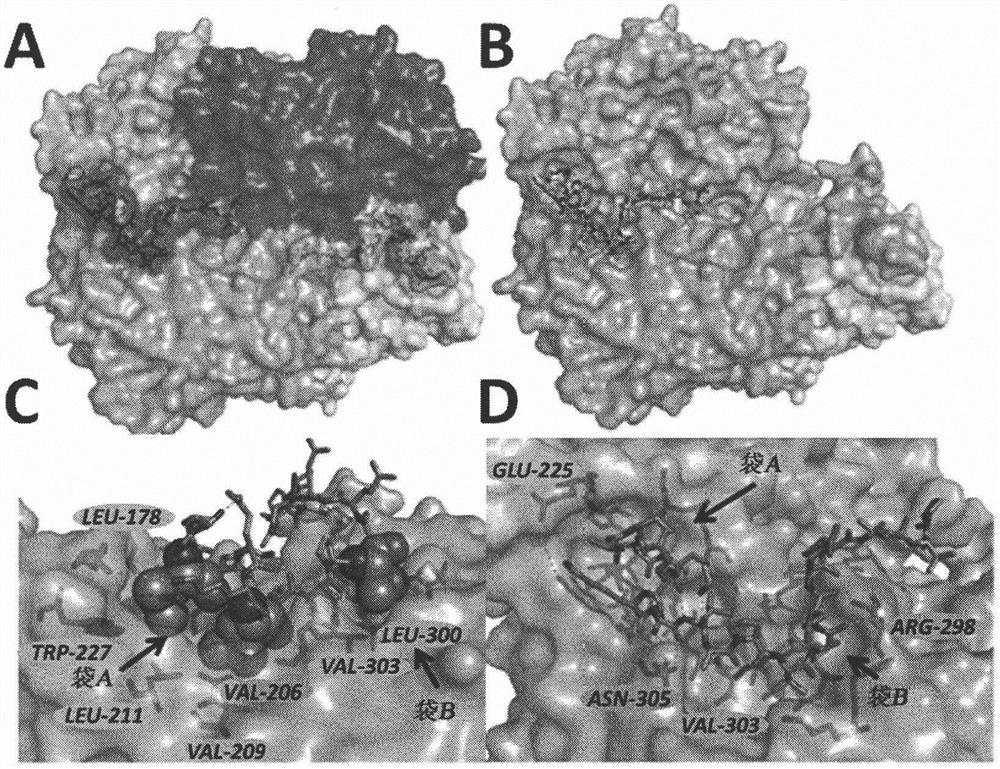
[0045] To establish a new way to inhibit LDH, the focus is on unraveling LDH allosteric sites, the targeting of which could lead to unprecedented approaches to this problem. Given that tetramer is the smallest functional unit, the activity of LDH depends on its catalytic site and oligomerization state. With regard to LDHs, since their N-terminal arms extend from one subunit and wrap around two adjacent subunits, the subunits are held together, thereby promoting cohesion of the overall tetramer. Interestingly, the 32 N-terminal amino acid fragment of LDH is known to interfere with LDH tetramerization in vitro ( et al. (1987)). Taken together, these observations prompted the inventors to evaluate this N-terminal arm as a starting point for the design and development of molecules that interfere with LDH tetramerization.
[0046] The present invention relates to polypeptides that modulate the activity of at least one isoform of native tetrameric lactate dehydrogenase.
[0047]...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap