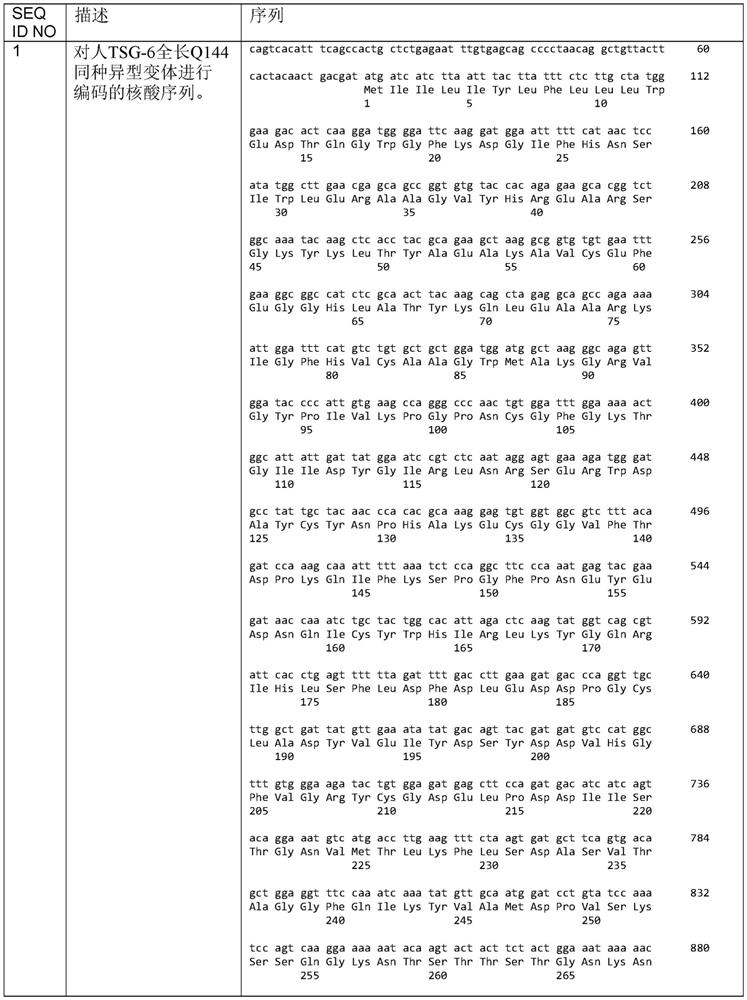
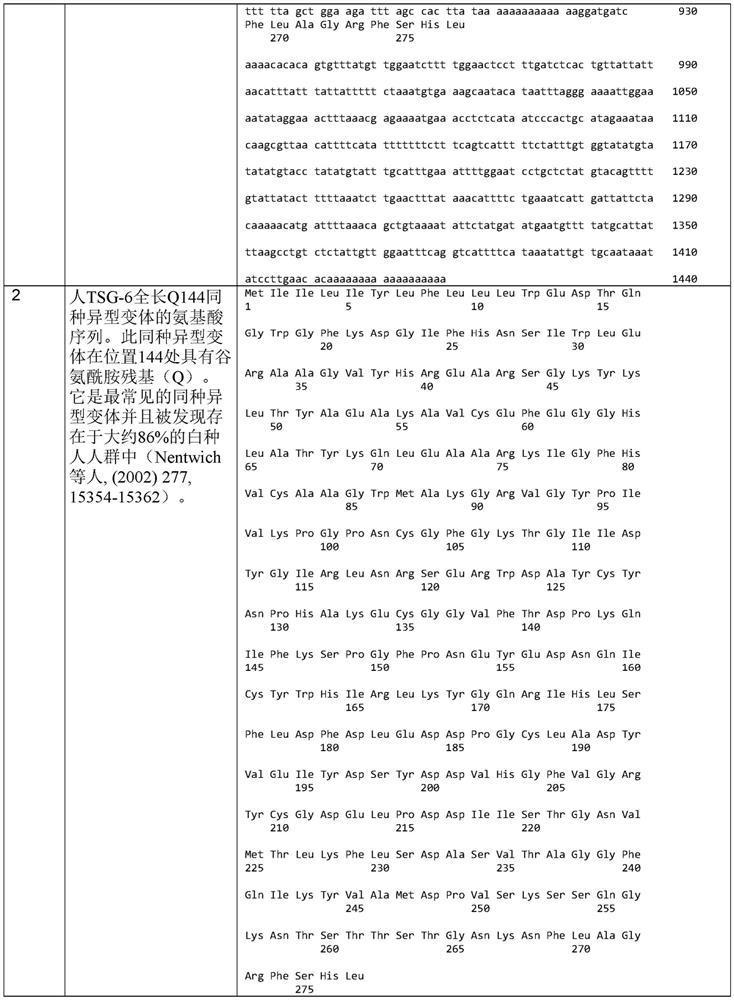
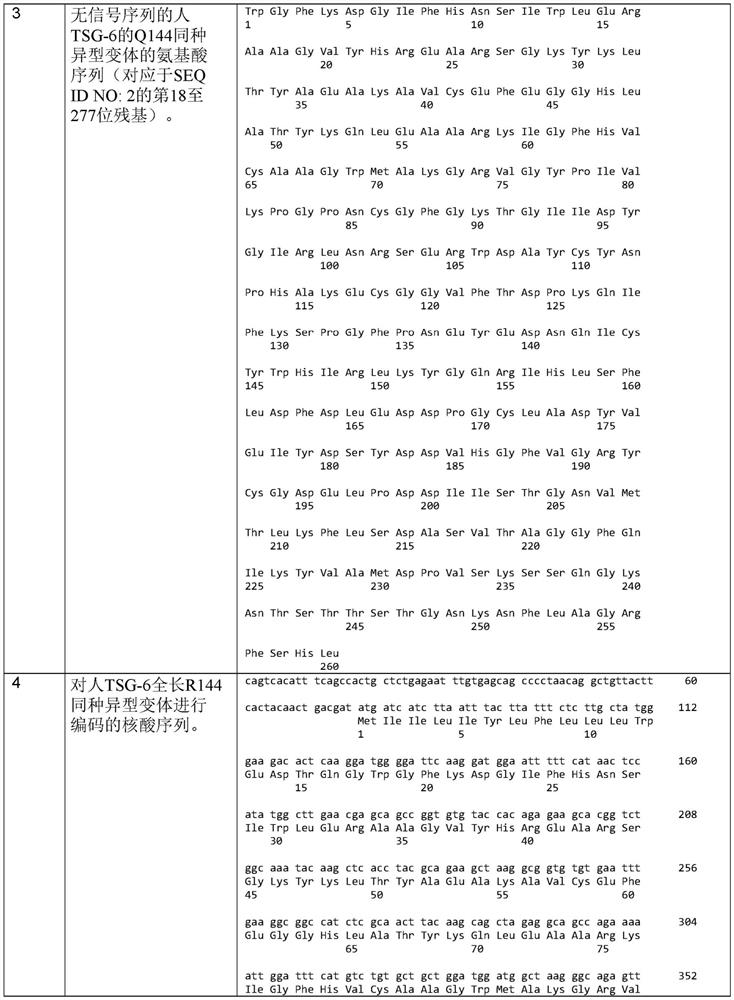
TSG6 polypeptide fragments for dry eye disease
A dry eye disease and disease technology, applied in the field of treatment of dry eye disease, can solve problems such as changes in the stability of recombinant proteins, difficulties in large-scale production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0137] Example 1: LINK_TSG6 is more soluble than full-length TSG-6
[0138] The solubility and aggregation state of full-length TSG-6 and LINK_TSG6 in PBS were compared over a range of concentrations using UV spectrophotometry and dynamic light scattering (DLS). LINK_TSG6 was completely dissolved at 2 mg / ml and showed no aggregation at 0.4, 0.8, 1.6 or 3.2 mg / ml. The solubility of the full-length protein was much lower, with less than 40% of the protein remaining in solution at 0.4, 0.8, 1.6 and 3.2 mg / ml. The full-length protein was also highly aggregated at 0.2, 0.40.8, 1.6 and 3.2 mg / ml. DLS measurements of full-length TSG-6 at 1.6 and 3.2 mg / ml were out of range, indicating the formation of aggregates greater than 25 million Daltons.
example 2
[0139] Example 2: LINK TSG6 Ameliorates Signs and Symptoms of Dry Eye Disease in a Mouse Model
[0140] Topical TSG-6 has previously been shown to be as effective as cyclosporine eye drops in inflammation-mediated dry eye disease (Kim et al., 2016). To test whether the LINK_TSG6 polypeptide could be used for this indication, we investigated its effect in the NOD.B10 mouse model of primary Sjögren's syndrome (spontaneous dry eye without diabetes).
[0141] Put NOD.B10.H2 b Mice (12 weeks old, Jackson Lab), a model of primary Sjögren's syndrome (dry eye without diabetes), were treated with LINK_TSG6 topical application for 7 days. C57BL / 6 mice were used as a negative control as they did not suffer from spontaneous dry eye.
[0142] Mice were treated topically with 1 μg LINK_TSG6 in 10 μl PBS, 4 times a day (QID) for 7 days. Zolazepam-Teletamine by intraperitoneal injection Mice were anesthetized by Virbac, Carros, France and 10 μl of LINK_TSG6 or PBS was administered usin...
example 3
[0156] Example 3: The effect of LINK TSG6 on dry eye disease is dose-dependent
[0157] We were interested to know whether the effect observed in Example 1 was dose-dependent. To this end, we applied topically QID 1, 0.1 or 0.01 μg of LINK_TSG6 in 5 μl PBS to 12-week-old NOD.B10.H2b mice (Jackson Laboratory) for 7 days.
[0158] Mice were randomly assigned to the following treatment groups:
[0159] 1) Group 1 (negative control group): C57BL / 6 mice, 12 weeks old (n=6, 12 eyes) untreated
[0160] 2) Group 2 (positive control group): NOD.B10 (n=8, 8 eyes)+PBS 5μl QID
[0161] 3) Group 3 (experimental group): NOD.B10 (n=8, 8 eyes) + LINK_TSG6 1 μg (in 5 μl PBS) QID
[0162] 4) Group 4 (experimental group): NOD.B10 (n=8, 8 eyes)+LINK_TSG6 0.1μg QID
[0163] 5) Group 5 (experimental group): NOD.B10 (n=8, 8 eyes)+LINK_TSG6 0.01μg QID
[0164] The effect of the treatment was determined as in Example 1.
[0165] like Figure 3A Corneal epithelial defects were quantified in Li...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com