Compositions and methods for treating autism spectrum disorders
A composition and mixture technology, applied in the direction of drug combination, medical raw materials derived from bacteria, pharmaceutical formulations, etc., can solve problems such as uncertainty
Pending Publication Date: 2022-06-21
FINCH THERAPEUTICS HLDG LLC
View PDF52 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
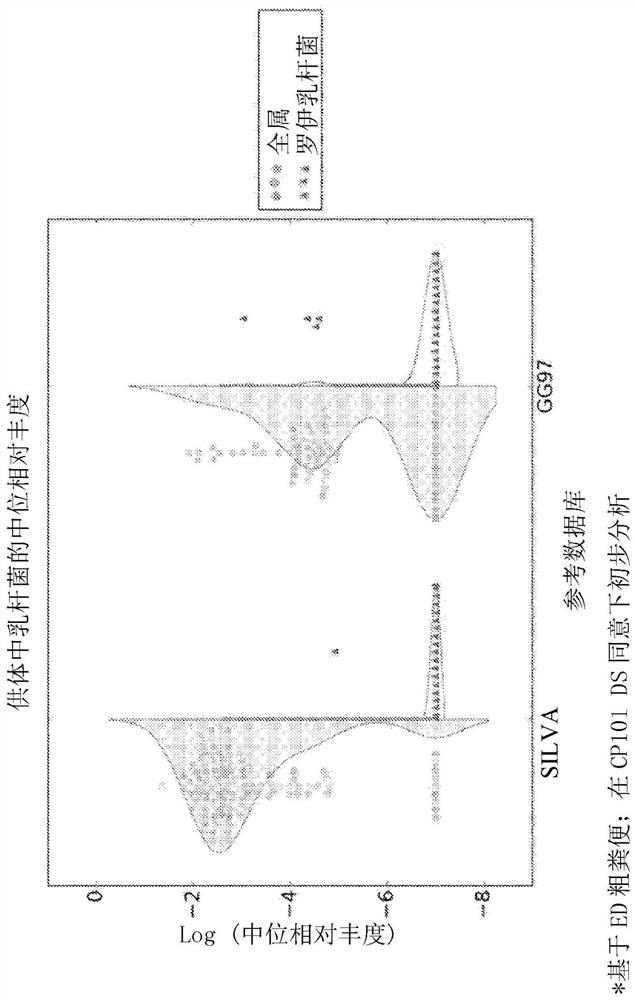
In addition, potential differences in the following could lead to uncertainty in the efficacy of fecal bacterial therapy in groups of patients with or predisposed to the disorder: (i) the identity and relative abundance of specific bacterial strains in different donor samples; and (ii) The degree of engraftment of specific bacterial strains in the gut of recipients of bacteriotherapy
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment approach 1
Embodiment approach 2
Embodiment approach 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
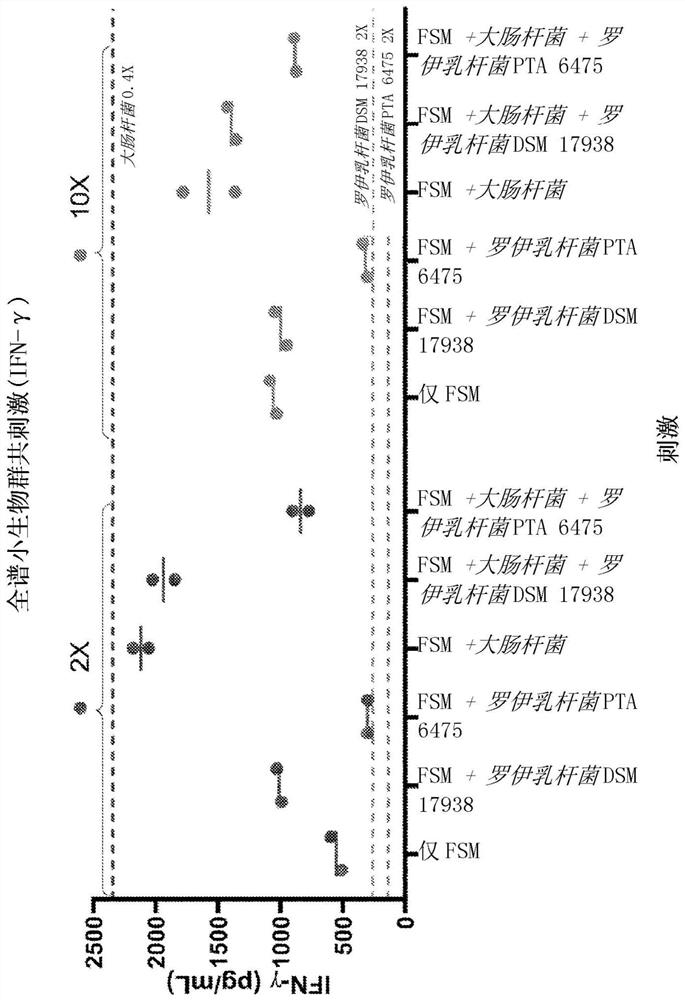
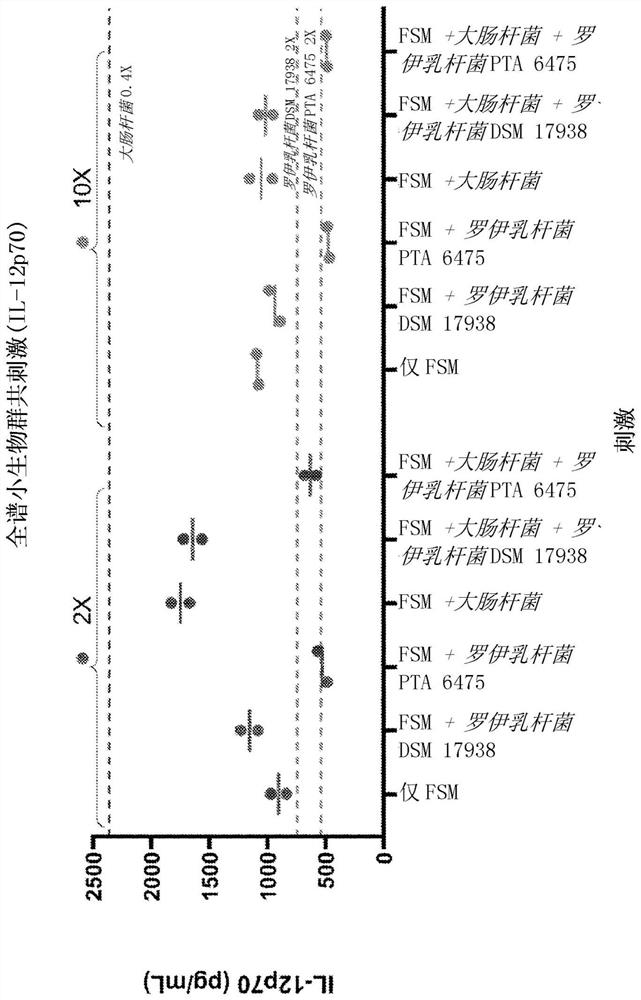
The present disclosure relates to compositions and methods for treating autism spectrum disorder (ASD). Provided herein are pharmaceutical compositions and formulations comprising an uncultured fecal bacterial product derived from fecal of a human donor and at least one, at least two or all three non-pathogenic microbial types selected from the group consisting of bacterial isolates, fungal isolates, and archaea isolates (e.g., bacterial isolates comprising Lactobacillus reuteri), and methods of treating ASD patients with the compositions. Further provided is a method of preparing a pharmaceutical composition comprising Lactobacillus reuteri.
Description
Cross References to Related Applications This application claims the benefit of U.S. Provisional Application No. 62 / 950,805, filed December 19, 2019, and U.S. Provisional Application No. 62 / 899,874, filed September 13, 2019, which are hereby incorporated by reference in their entirety. Background technique Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder characterized by widespread social and communication abnormalities, as well as restricted interests and repetitive behaviors. ASD typically presents in the first three years of life and manifests as characteristic symptoms or behavioral traits. The diagnosis of ASD now includes several conditions that were previously diagnosed separately: autism, combined developmental disorder not otherwise specified (PDD-NOS), and Asperger syndrome. All of these conditions are now covered by the diagnostic criteria for autism spectrum disorder specified in the American Psychiatric Association's Diagnostic & Statisti...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K35/747A61P25/00A61K35/74
CPCA61K35/74A61K35/747A61P25/00A61K45/06A61P25/18A61K2300/00
Inventor M·史密斯C·维登迈尔
Owner FINCH THERAPEUTICS HLDG LLC
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com