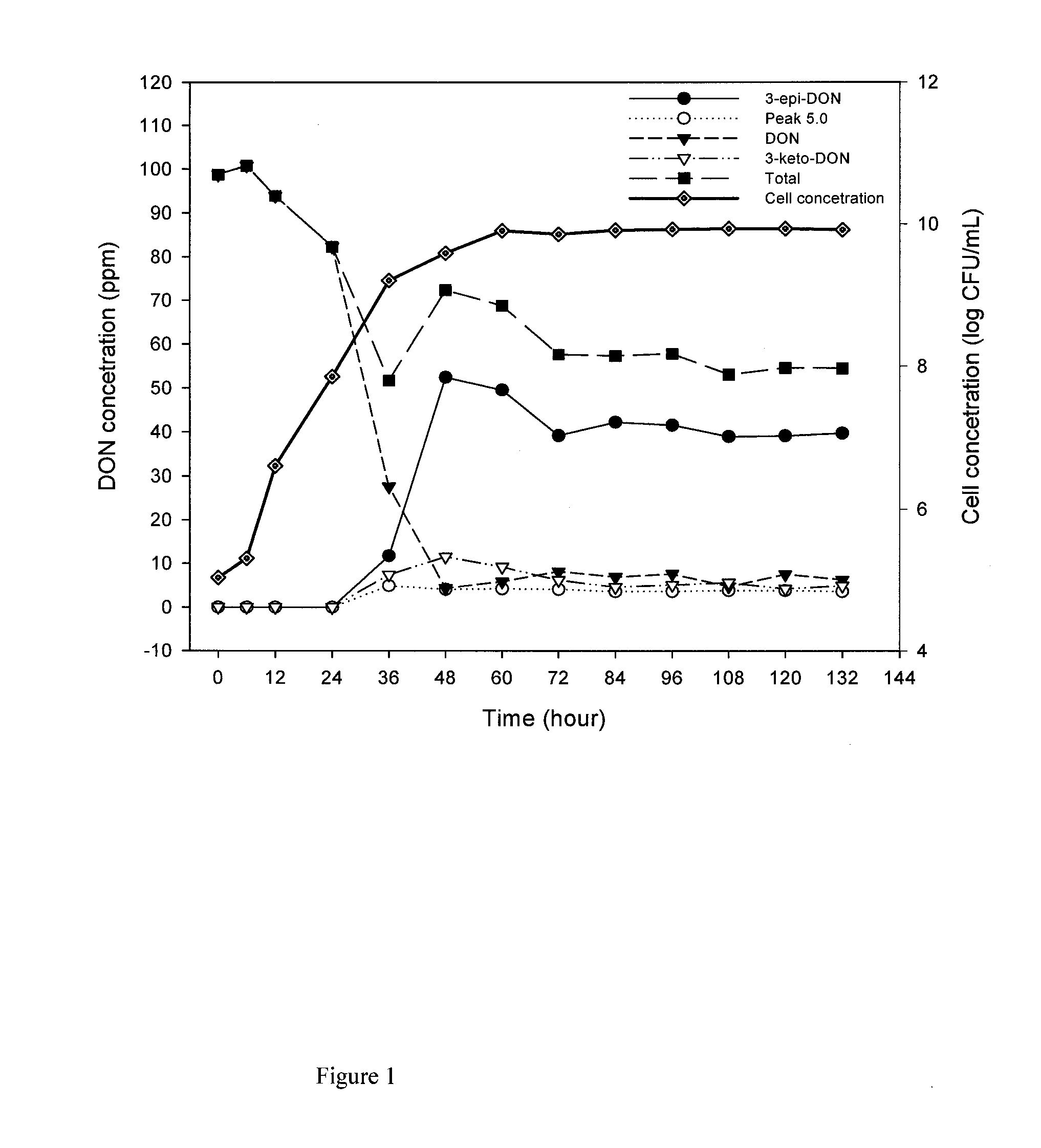
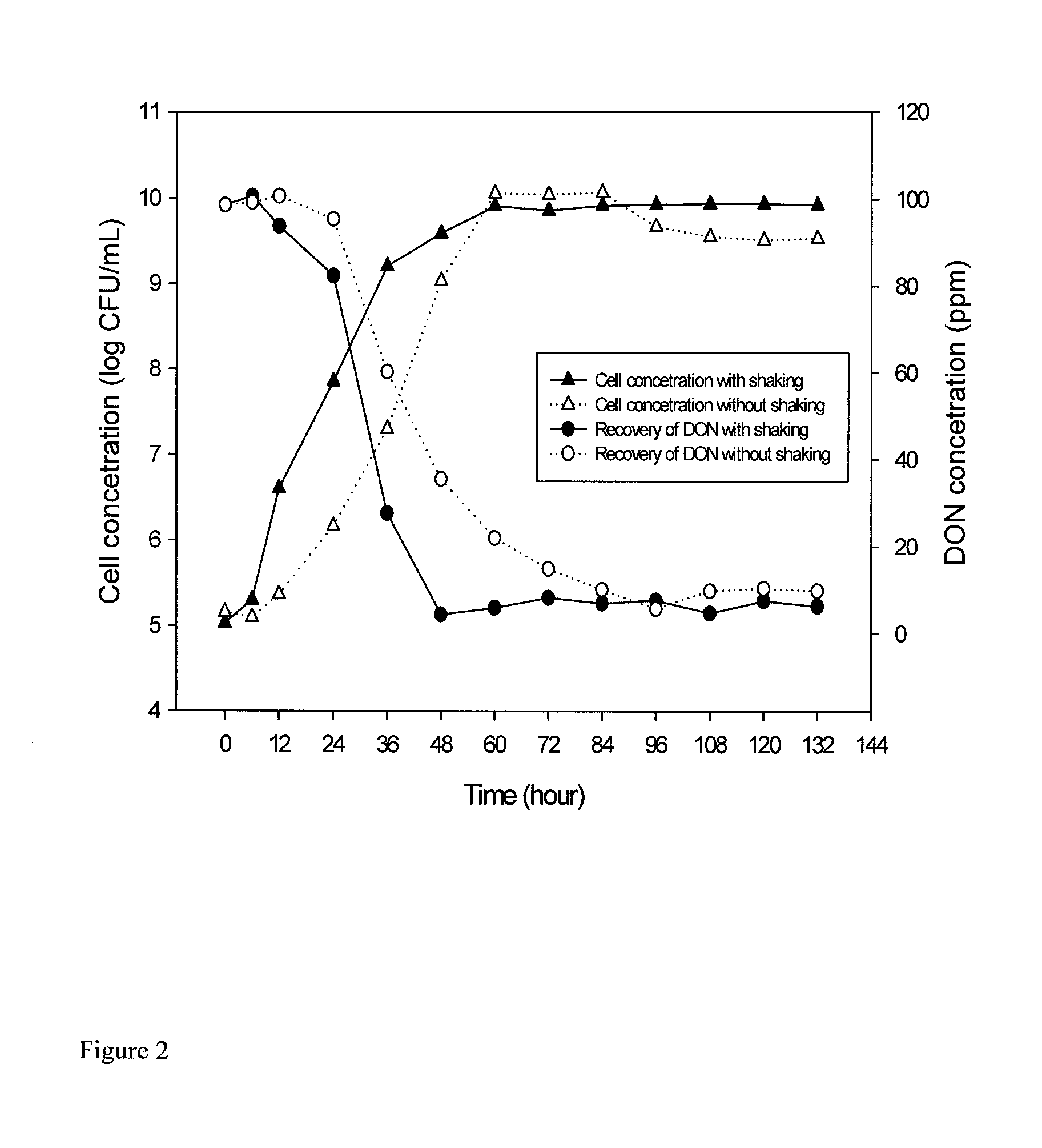
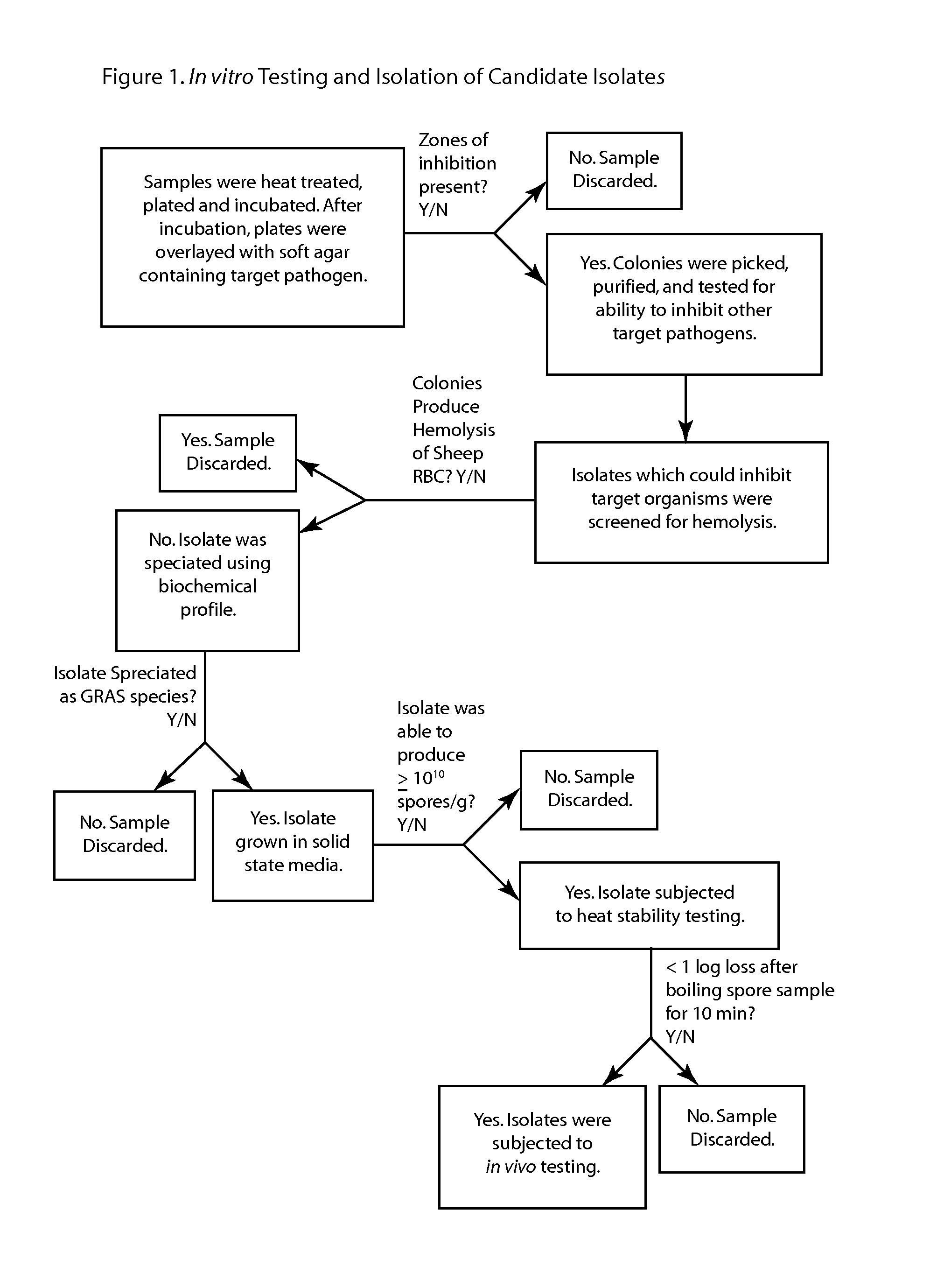
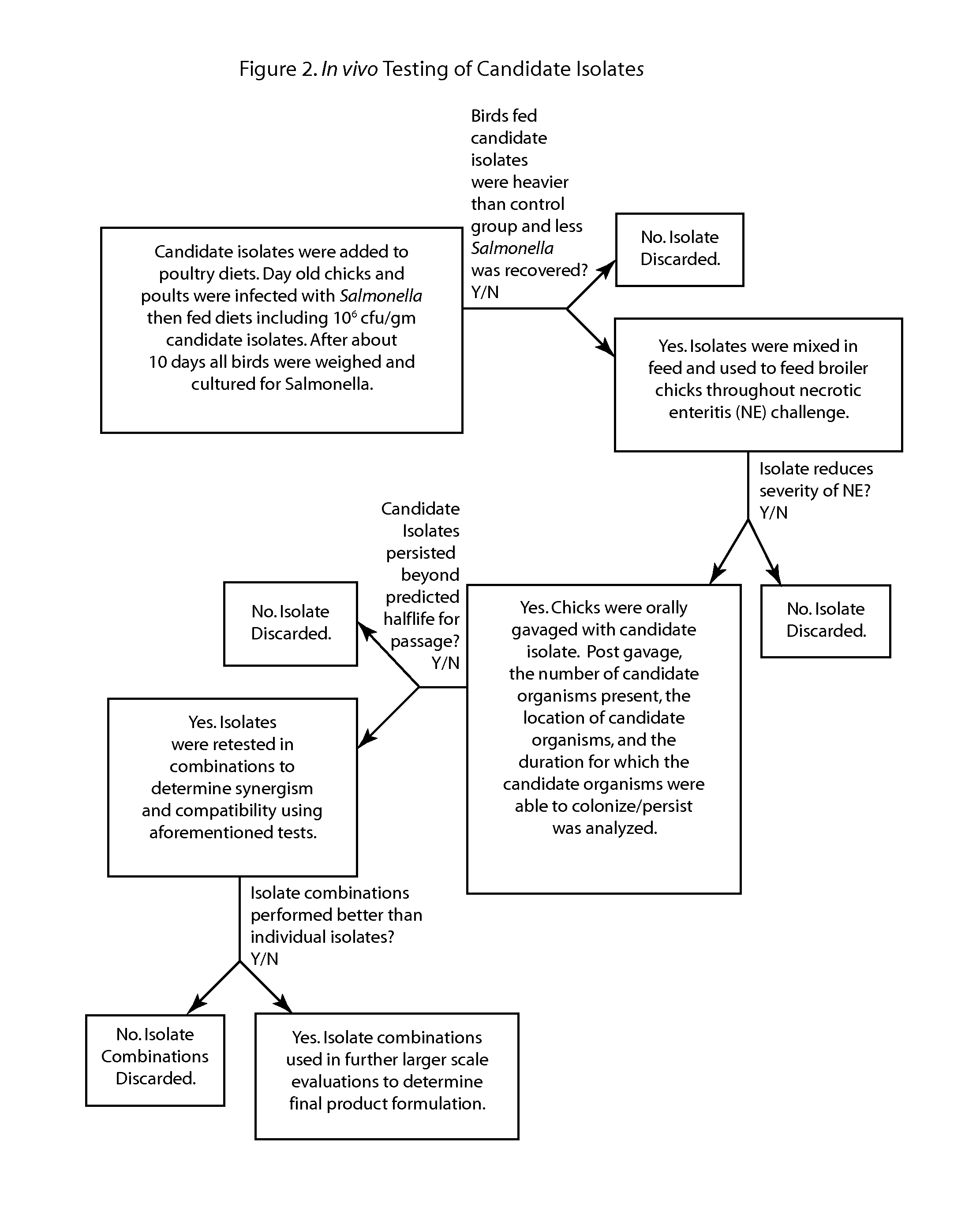
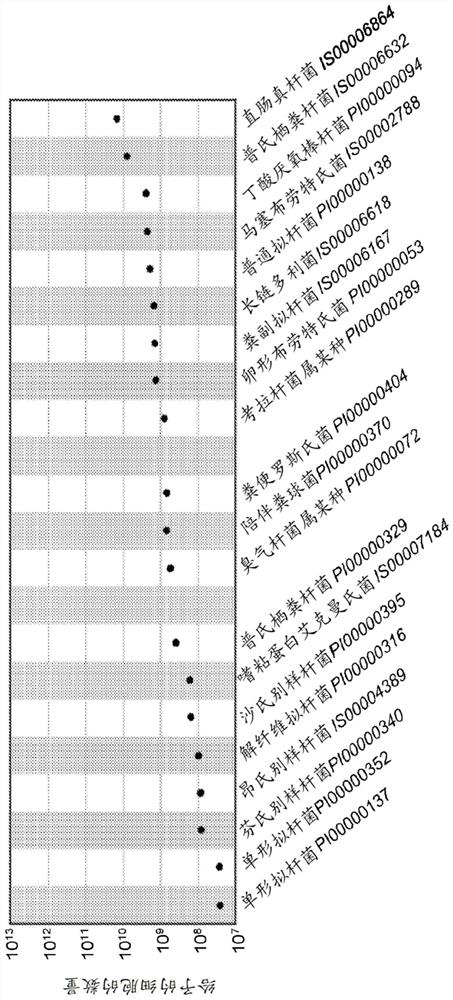
Patents
Literature
50 results about "Bacterial isolate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A: The purpose of streaking bacteria for isolation is to create a region in which the bacteria are so dilute that when each bacterium touches the surface of the agar, it is far enough away from other cells so that an isolated colony can develop.
Control of acidosis
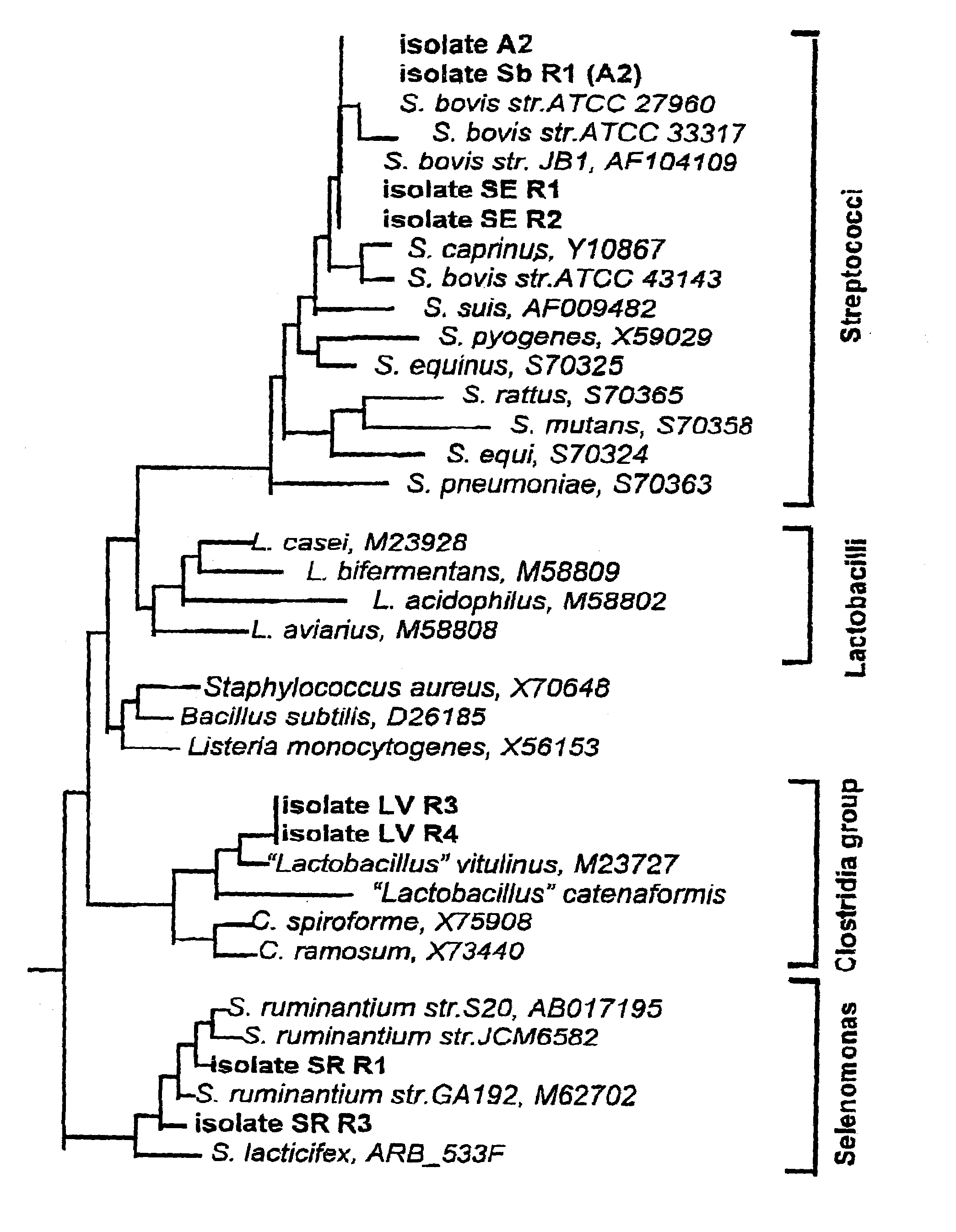
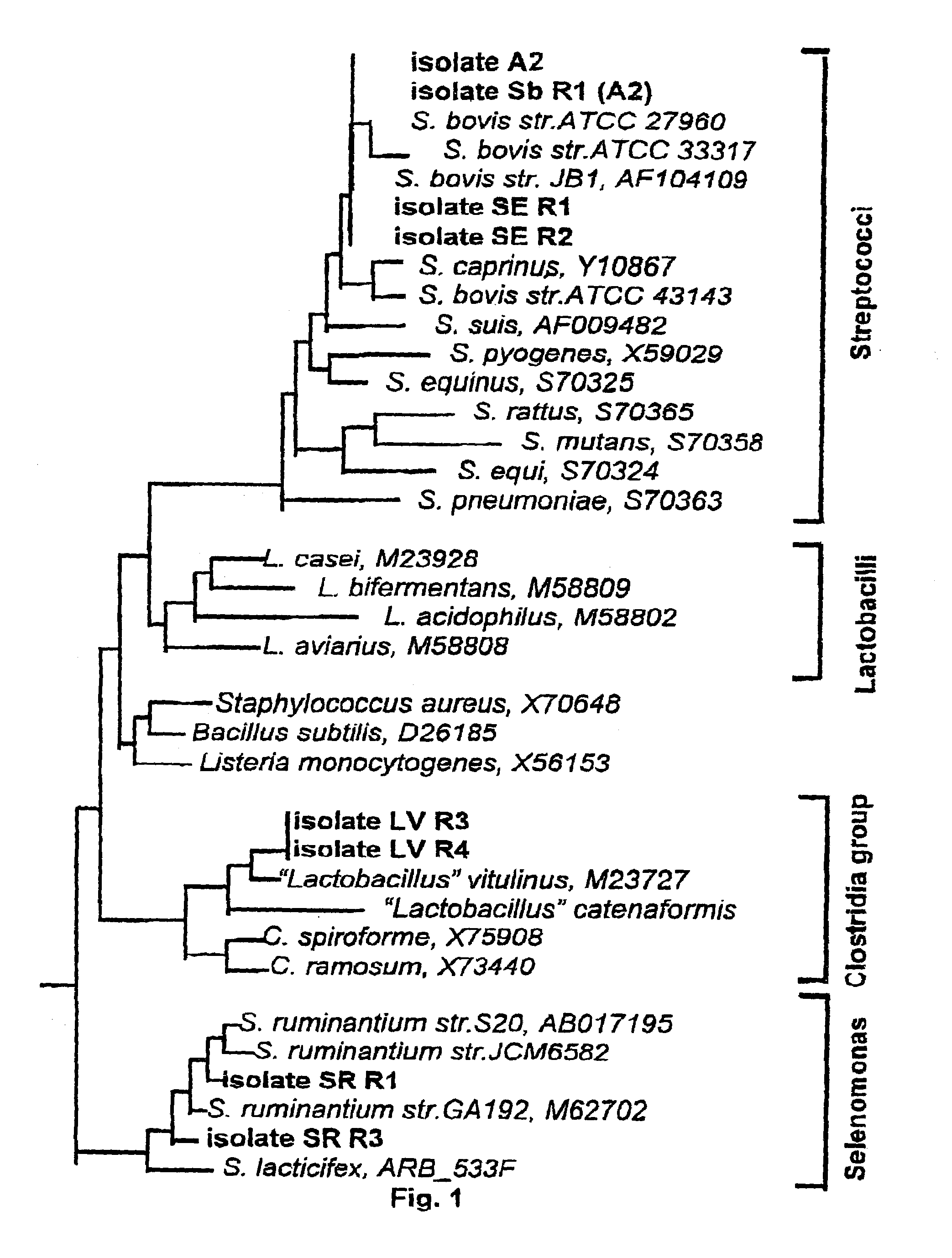
InactiveUS7011826B1Enhance immune responseBetter establishment of favourable starch utilising organismsOrganic active ingredientsBacteriaBiotechnologyBacteroides
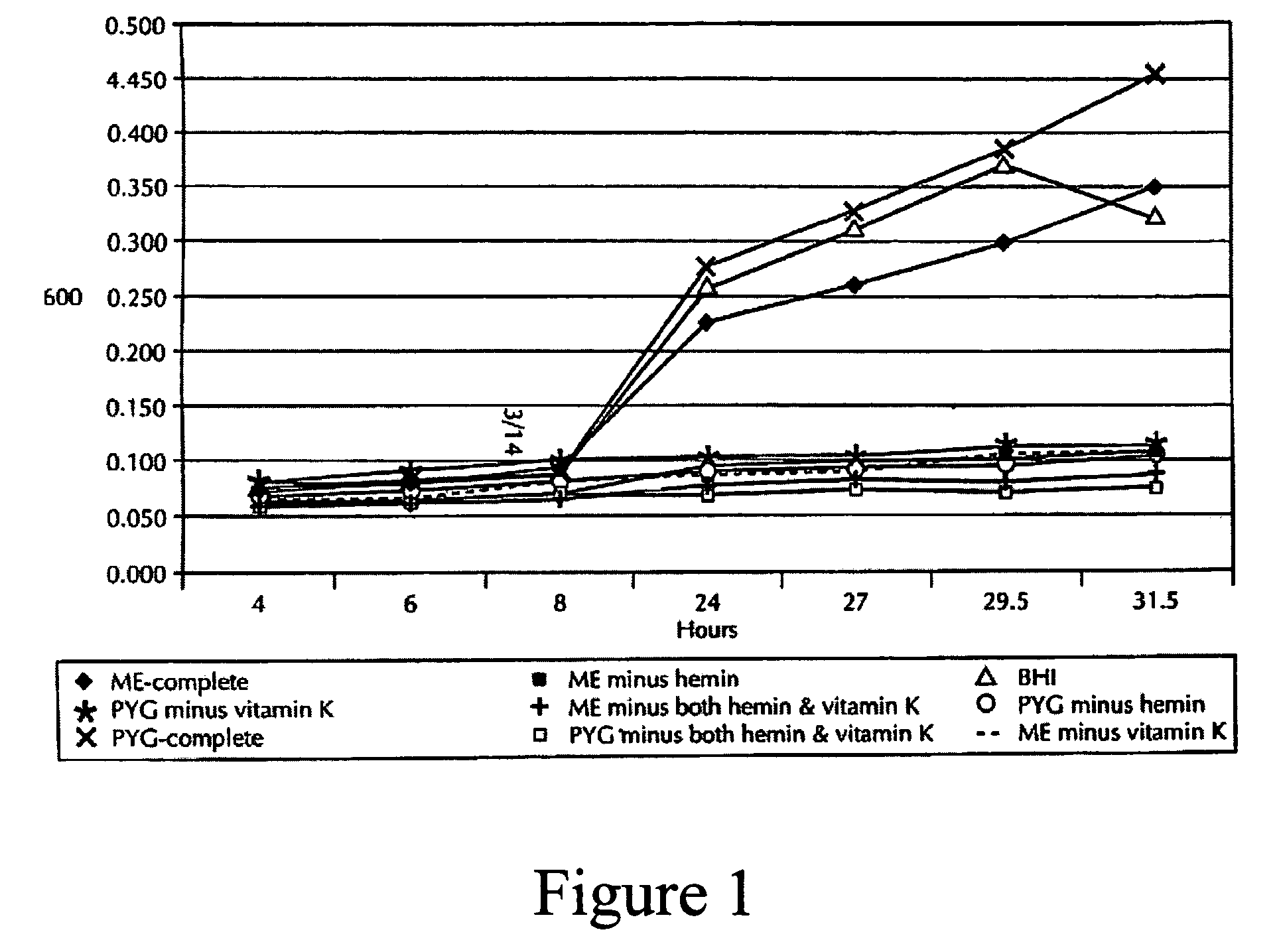
The present invention relates to a vaccine for the prevention of lactic acidosis in a vertebrate, said vaccine comprising at least one isolated microorganism, or fragment or fragments thereof, wherein said microorganism is capable of producing lactic acid within the gut of said vertebrate, and wherein said microorganism is selected from the group consisting of: Clostridium-like species, Prevotella-like species, Bacteroides-like species, Enterococcus-like species, Selenomonas species, non-dextran slime producing Streptococcus species and non-slime producing lactic acid bacterial isolates.
Owner:SPRUSON & FERGUSON +1
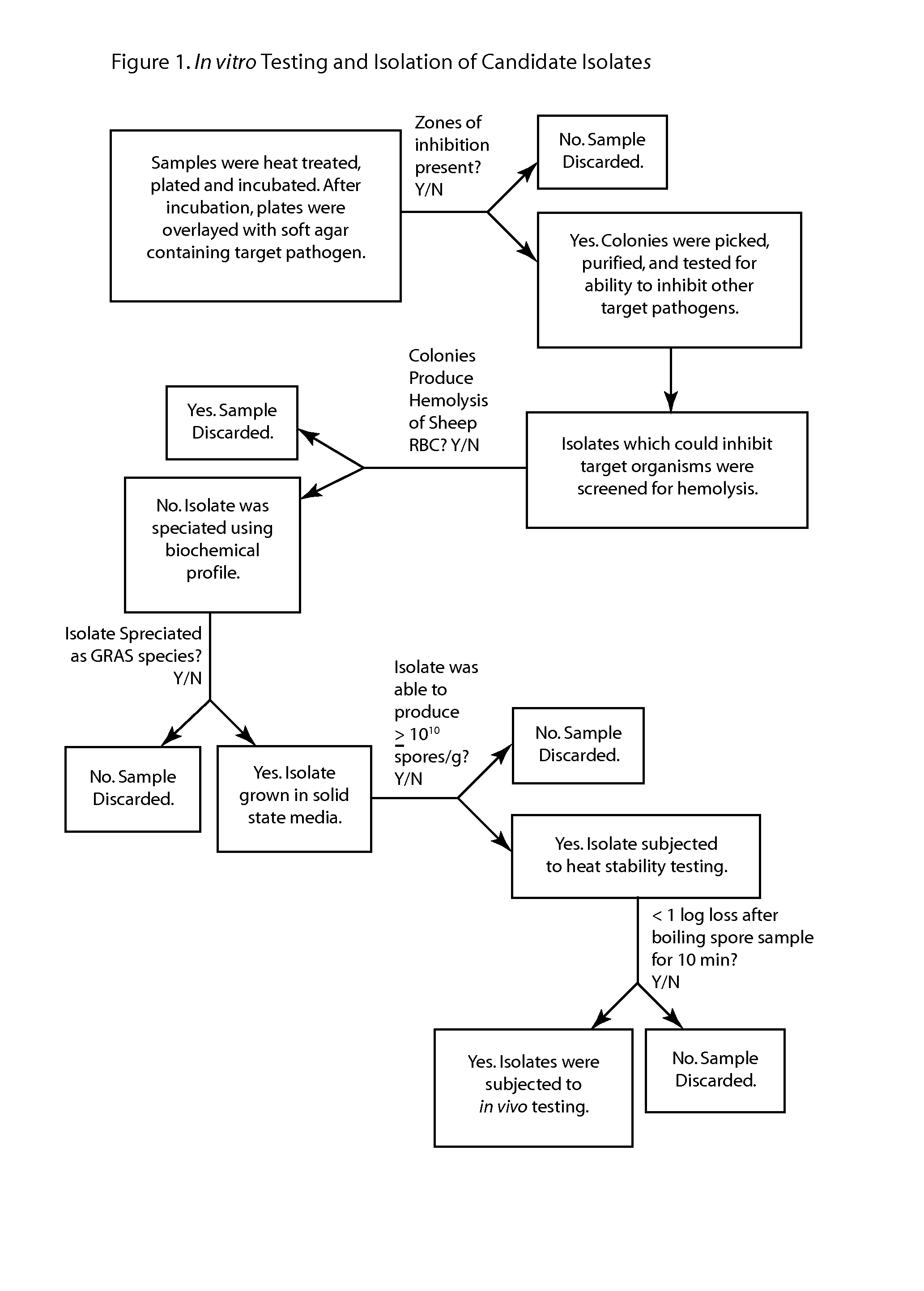
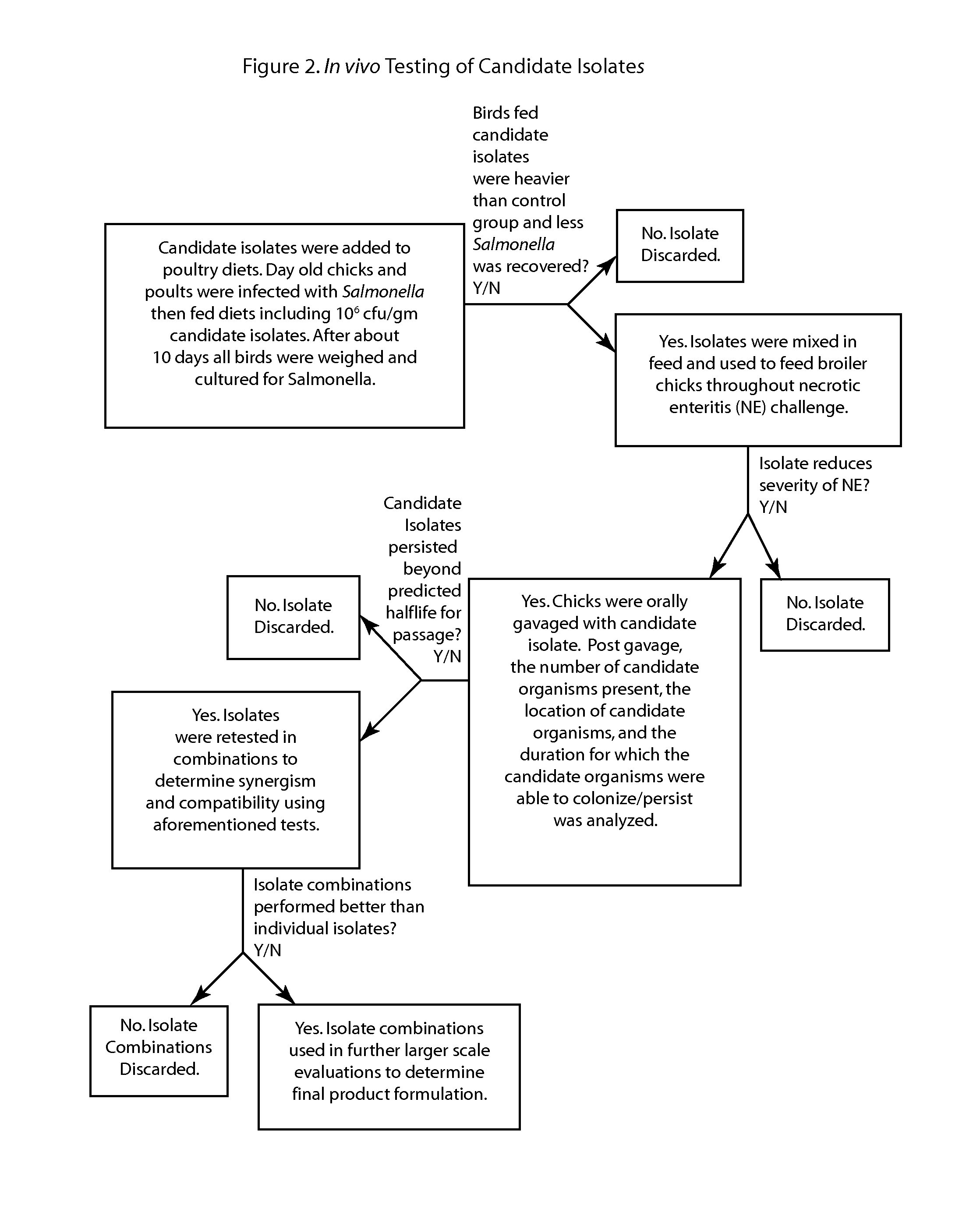
Methods and compositions including spore-forming bacteria for increasing the health of animals
ActiveUS20130136695A1Good for healthIncrease productionAntibacterial agentsBiocideBacteroidesBiology
Methods, compositions and bacterial isolates for improving the gastrointestinal health of animals and in particular of poultry are provided herein. The methods include administering an endospore-forming bacteria to an animal. The bacteria are selected for the ability to reduce the growth and presence of bacterial pathogens, such as Salmonella, Clostridium, and Campylobacter, in the gastrointestinal tract of the animal. The bacteria are also selected for the ability to improve at least one production parameter in the animal.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Kit for detecting klebsiella pneumoniae
ActiveCN104946762ASimplify testing proceduresShort detection cycleMicrobiological testing/measurementDNA/RNA fragmentationK pneumoniaeNucleotide
The invention discloses a kit for detecting klebsiella pneumoniae, and belongs to the technical field of PCR detection. The kit comprises specific primers and a probe for detecting klebsiella pneumoniae; the nucleotide sequences of the specific primers and the probe are as shown in SEQ ID NO.1-3; a detected target gene is a sequence of a pho gene, and has a nucleotide sequence as shown in SEQ ID No.4. The kit has the advantages of being accurate in detection, high in sensitivity, strong in specificity, and simple and quick to operate, has a favorable sample detection capacity, can replace a traditional bacterial isolated cultivation and diagnosis method, and the novel kit for quick detection is provided for klebsiella pneumoniae.
Owner:BIOSINO BIO TECH & SCI
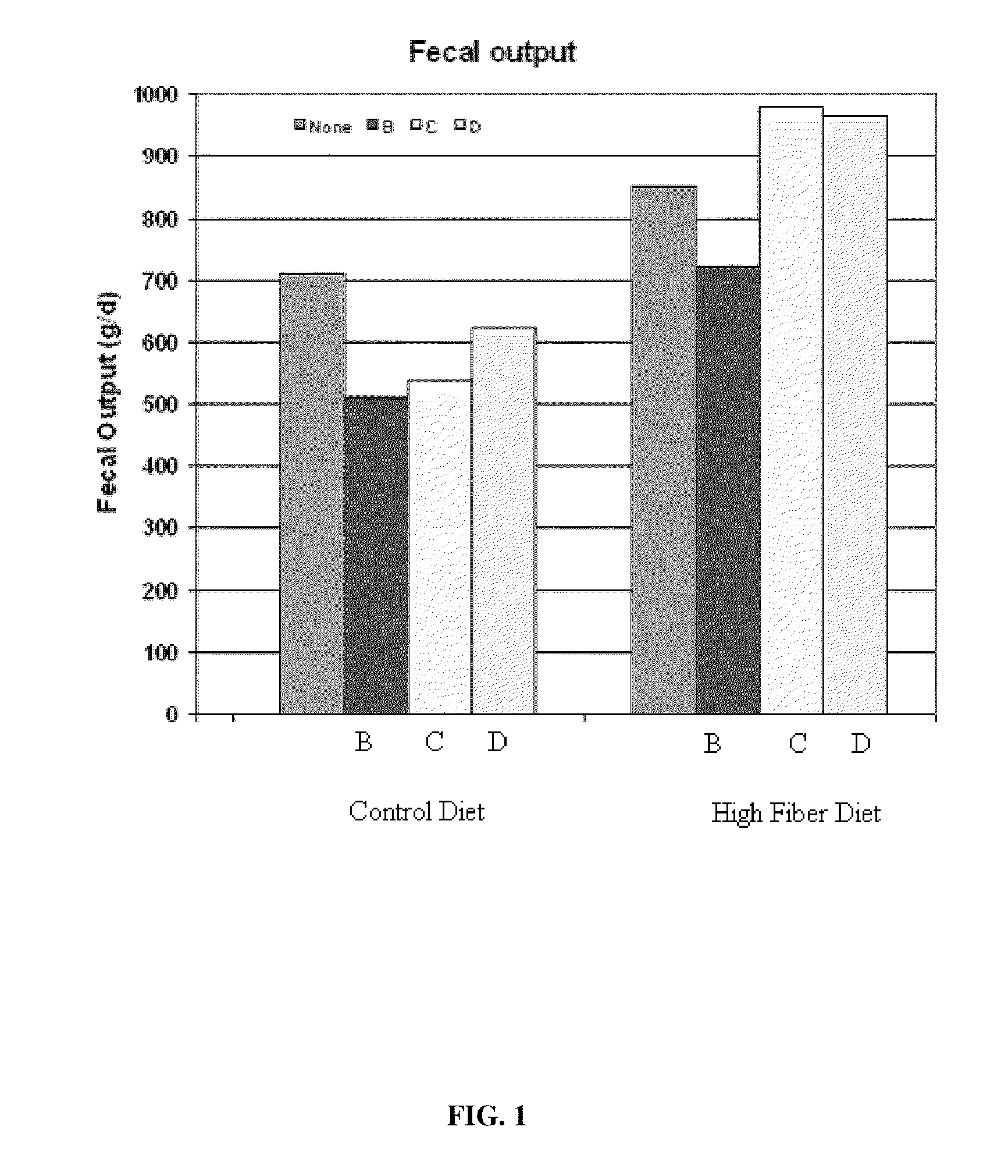
Novel Fibro-Biotic Bacterium Isolate
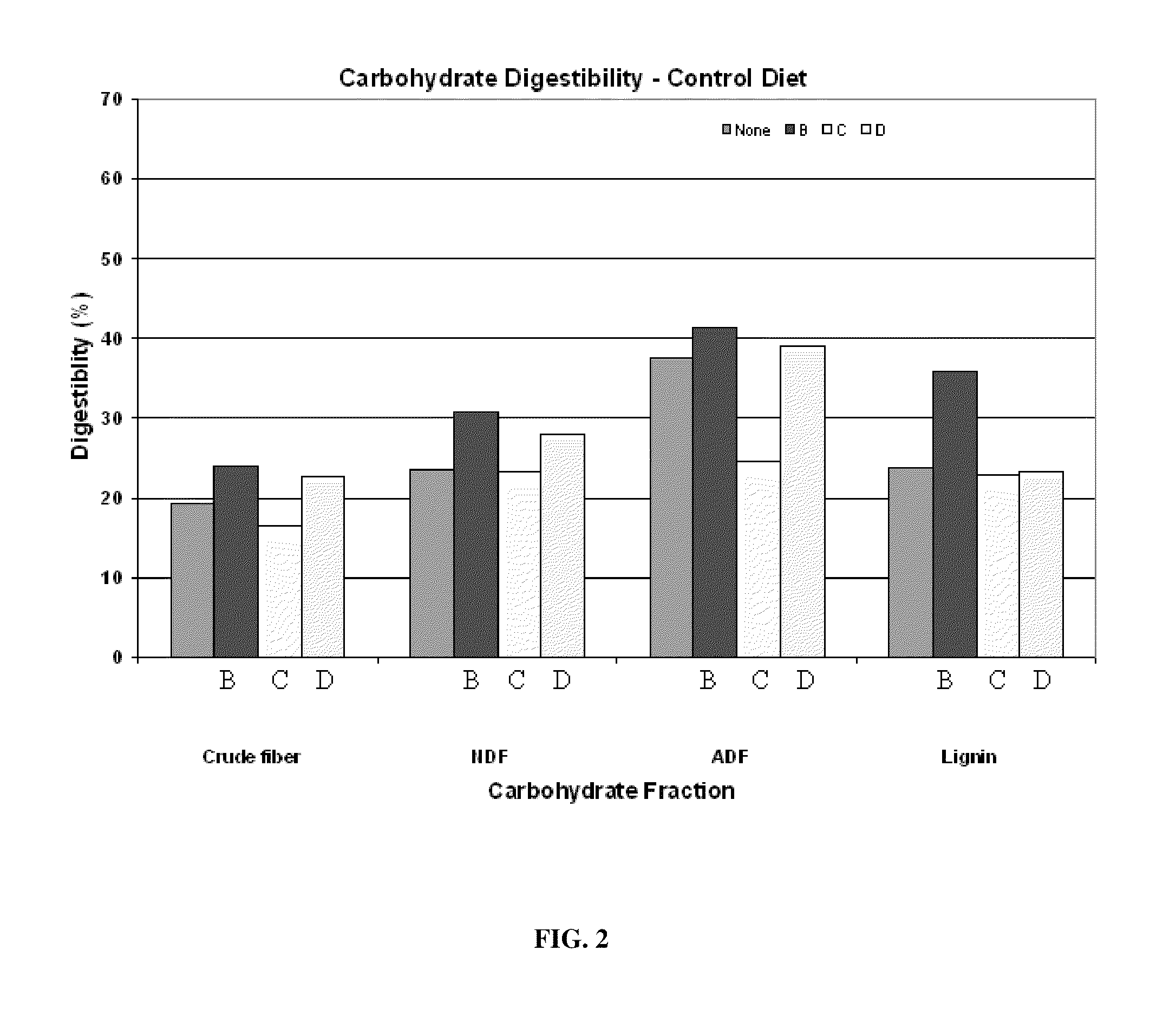
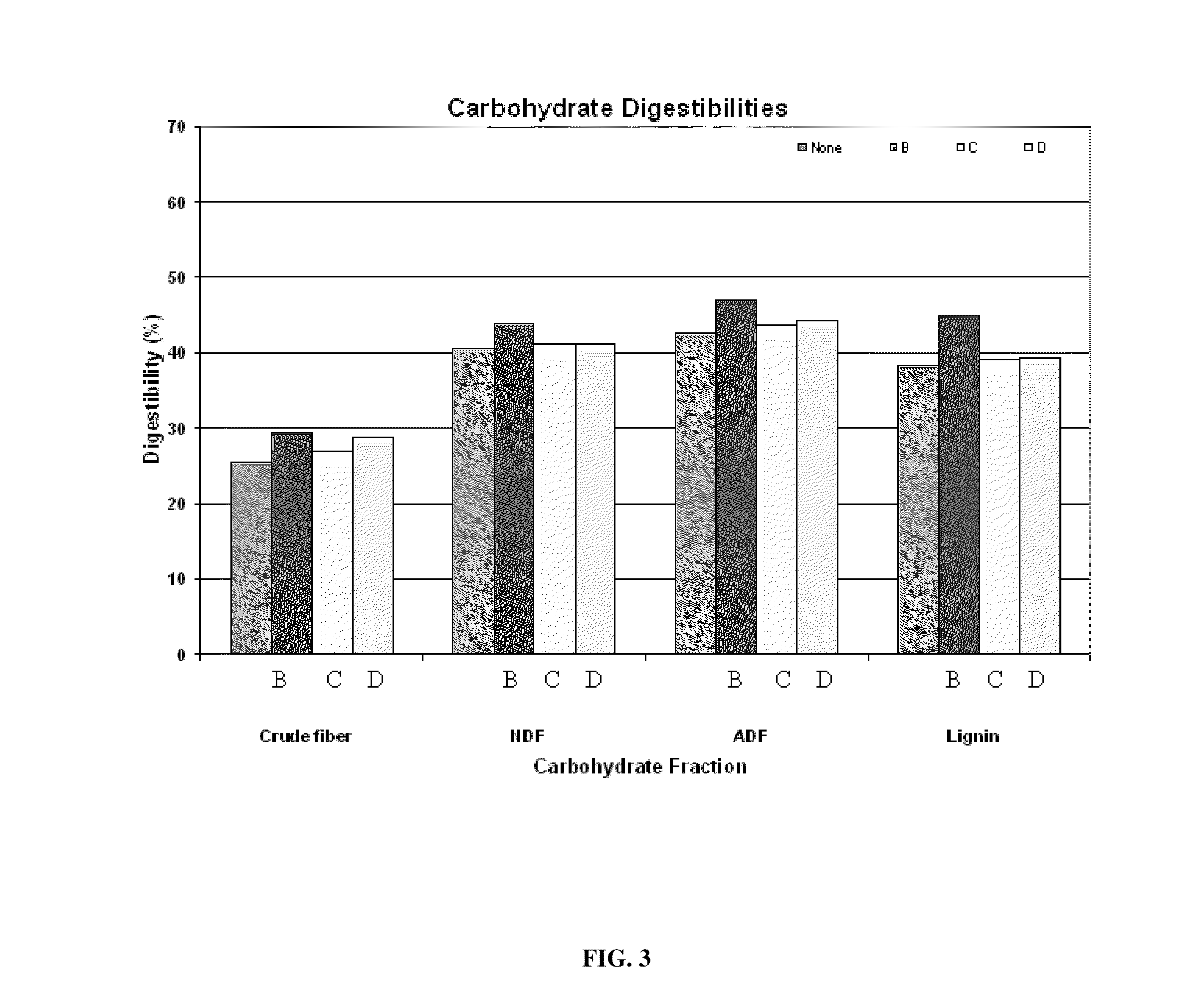
InactiveUS20110076356A1Improving fiber digestionReduce fecal outputMilk preparationBacteriaBiotechnologyFiber
The present invention relates to an isolated anaerobic bacterium Bacteroides sp. for use as a fibro-biotic for livestock. More specifically, the fibro-biotic supplements a fiber diet to Sus scrofa scrofa with the fibro-biotic improving fiber digestion and decreasing fecal output in these livestock. The present invention also relates to the use of the isolated bacterium as a food composition to improve the health of monogastric livestock and as a feed supplement to livestock.
Owner:GEORGIA TECH RES CORP +1
One-step multiplex pcr for the identifiation and differentiation of campylobacter species
InactiveUS20060051752A1Sugar derivativesMicrobiological testing/measurementPcr assayBacterial isolate
Described herein are a plurality of primers which may be used in a multiplex PCR assay in a fast, accurate, reliable and specific fashion for detecting the presence of specific Campylobacter strains within a sample. These kits can be used on bacterial isolates and has the potential for use directly on foods and environmental samples.
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA REPRESENTED BY THE MIN OF HEALTH
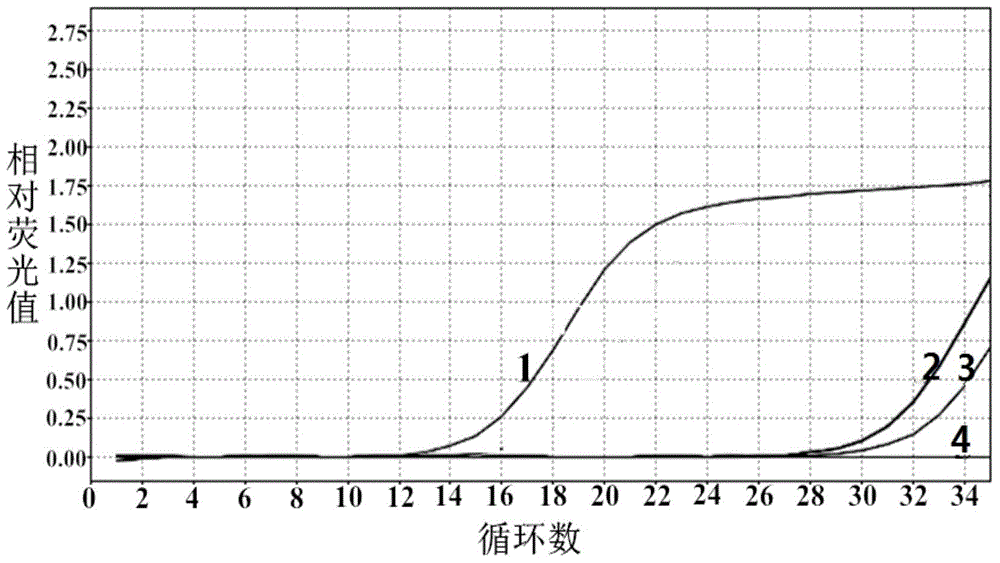
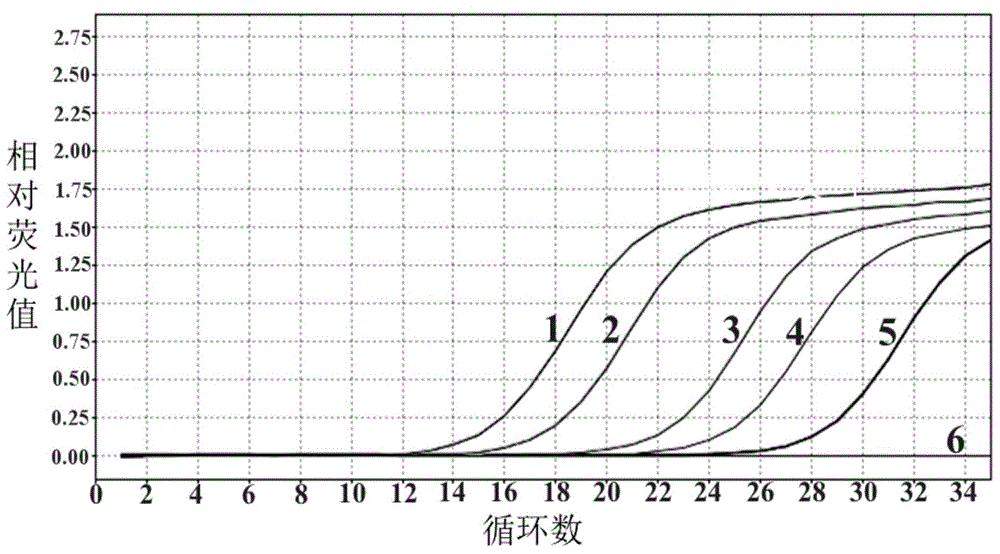
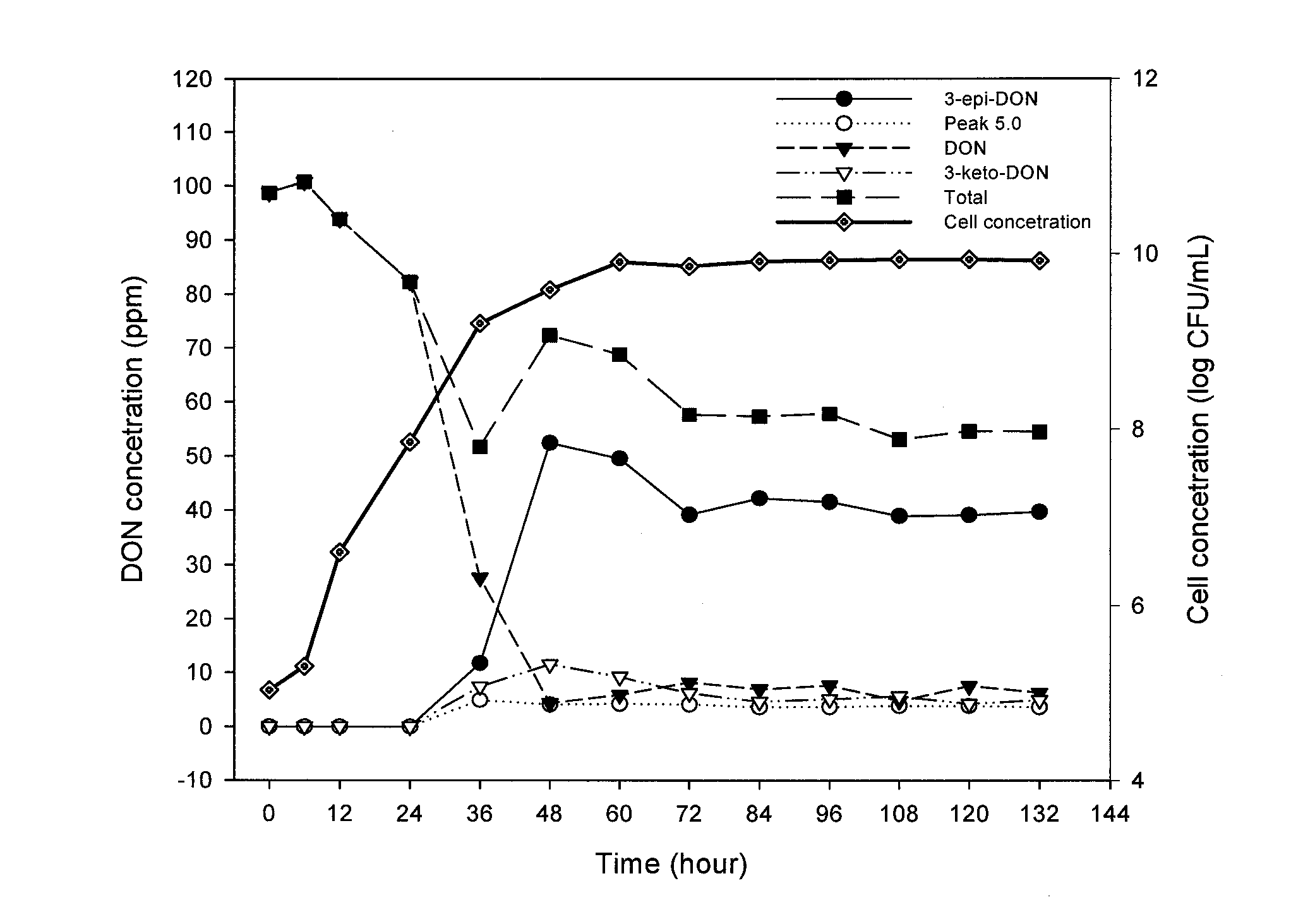
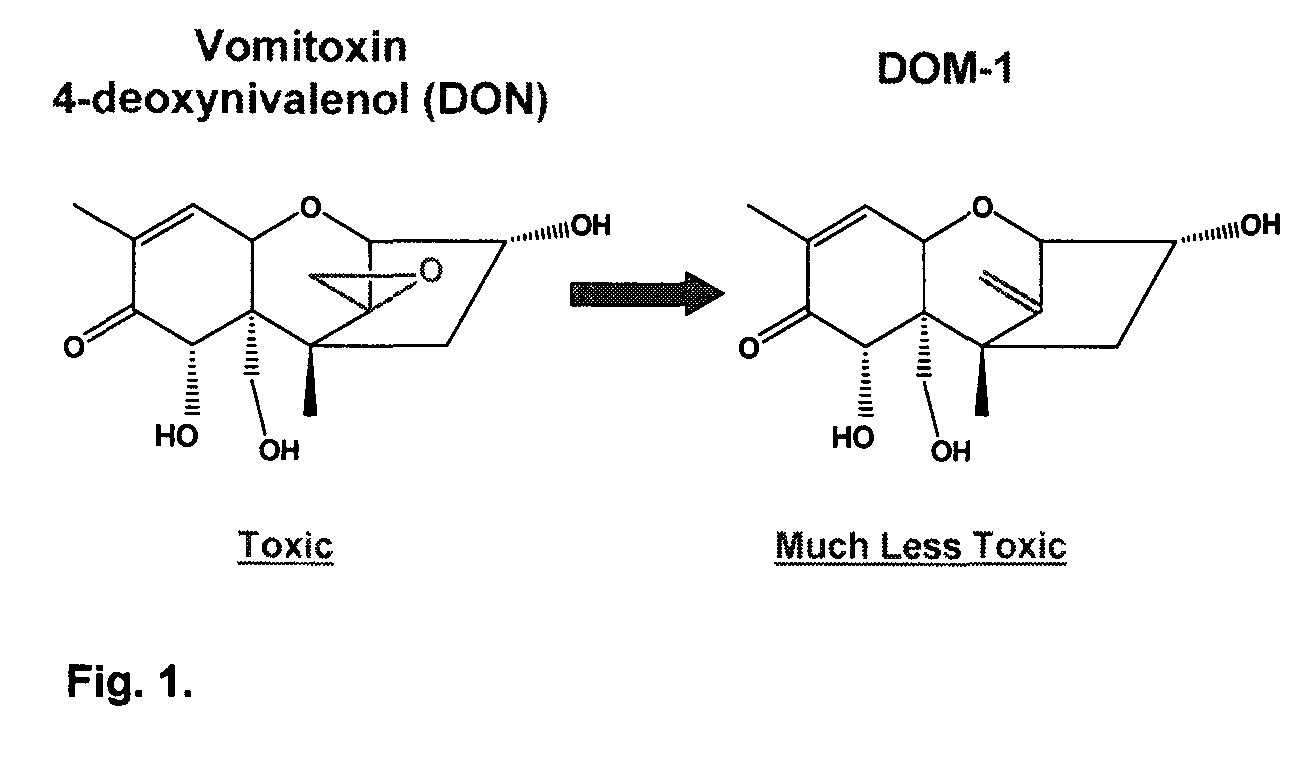
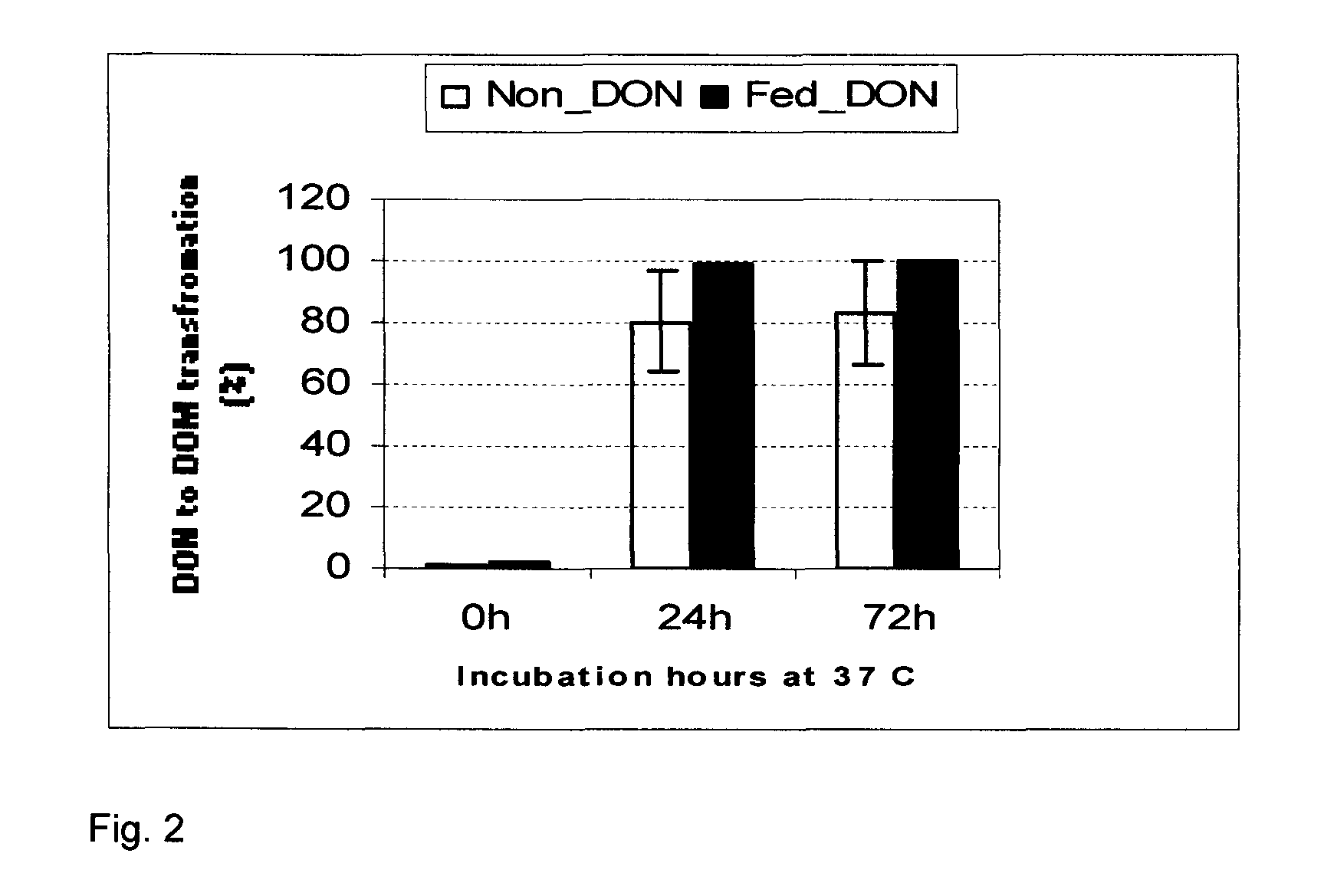
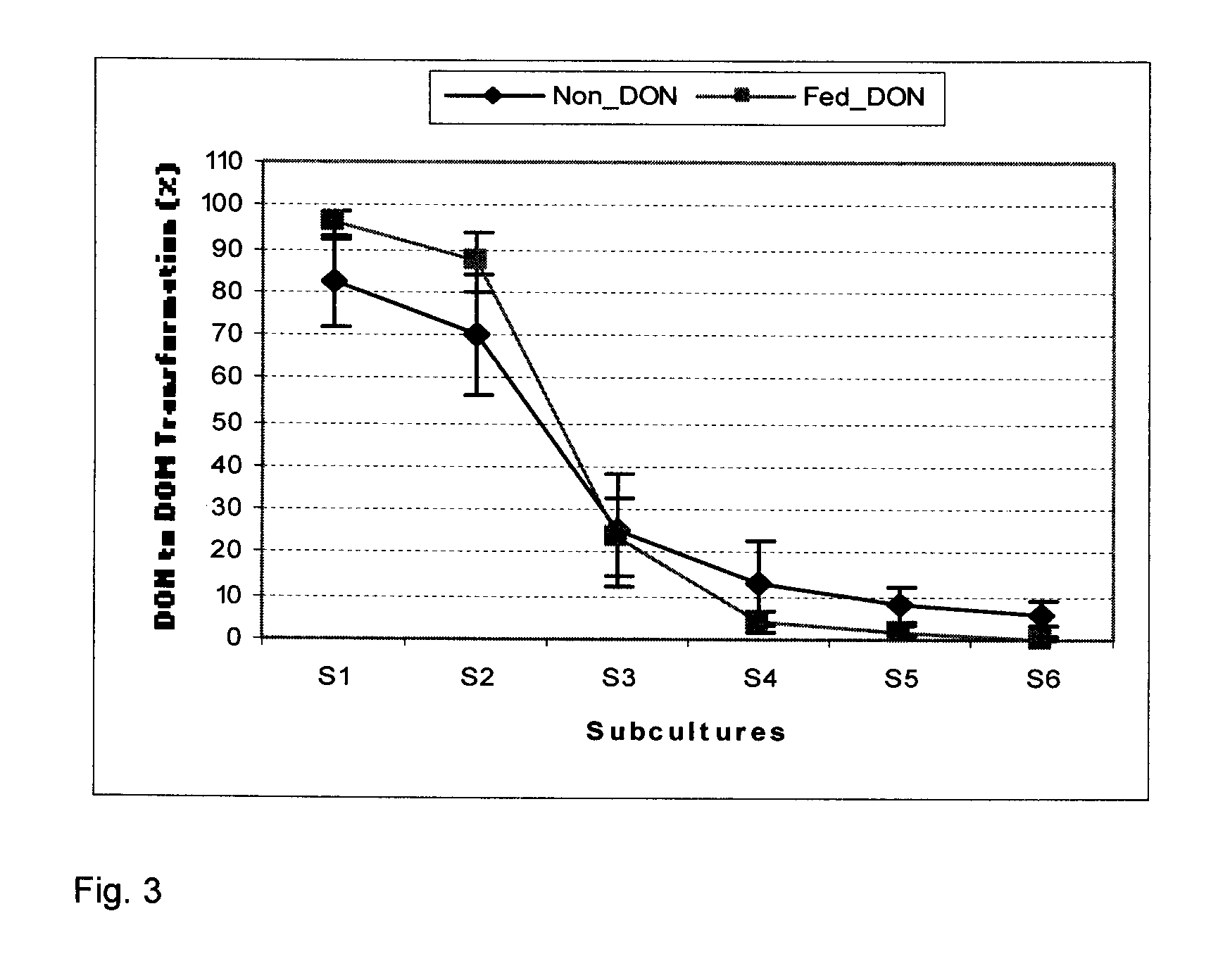
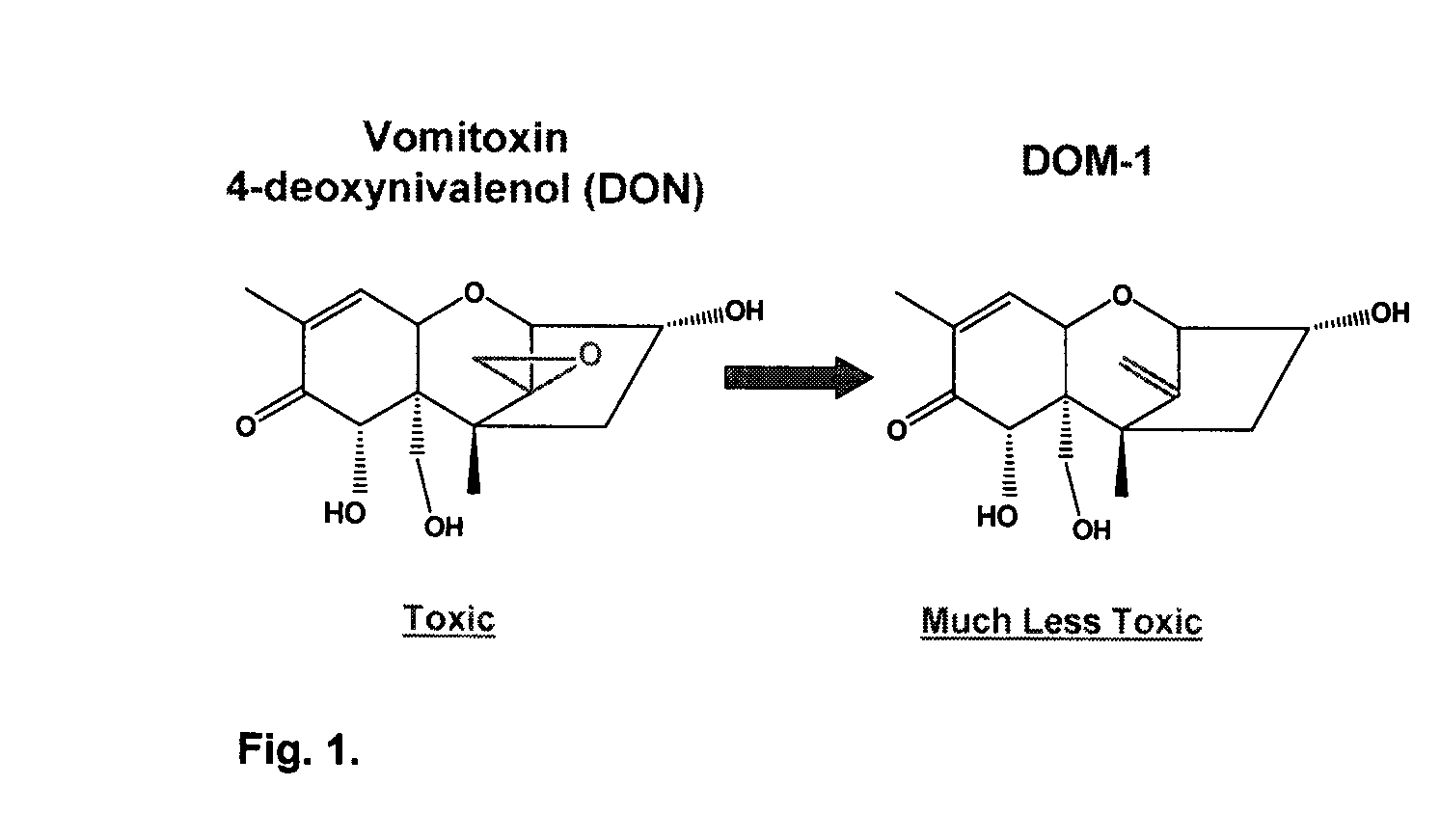
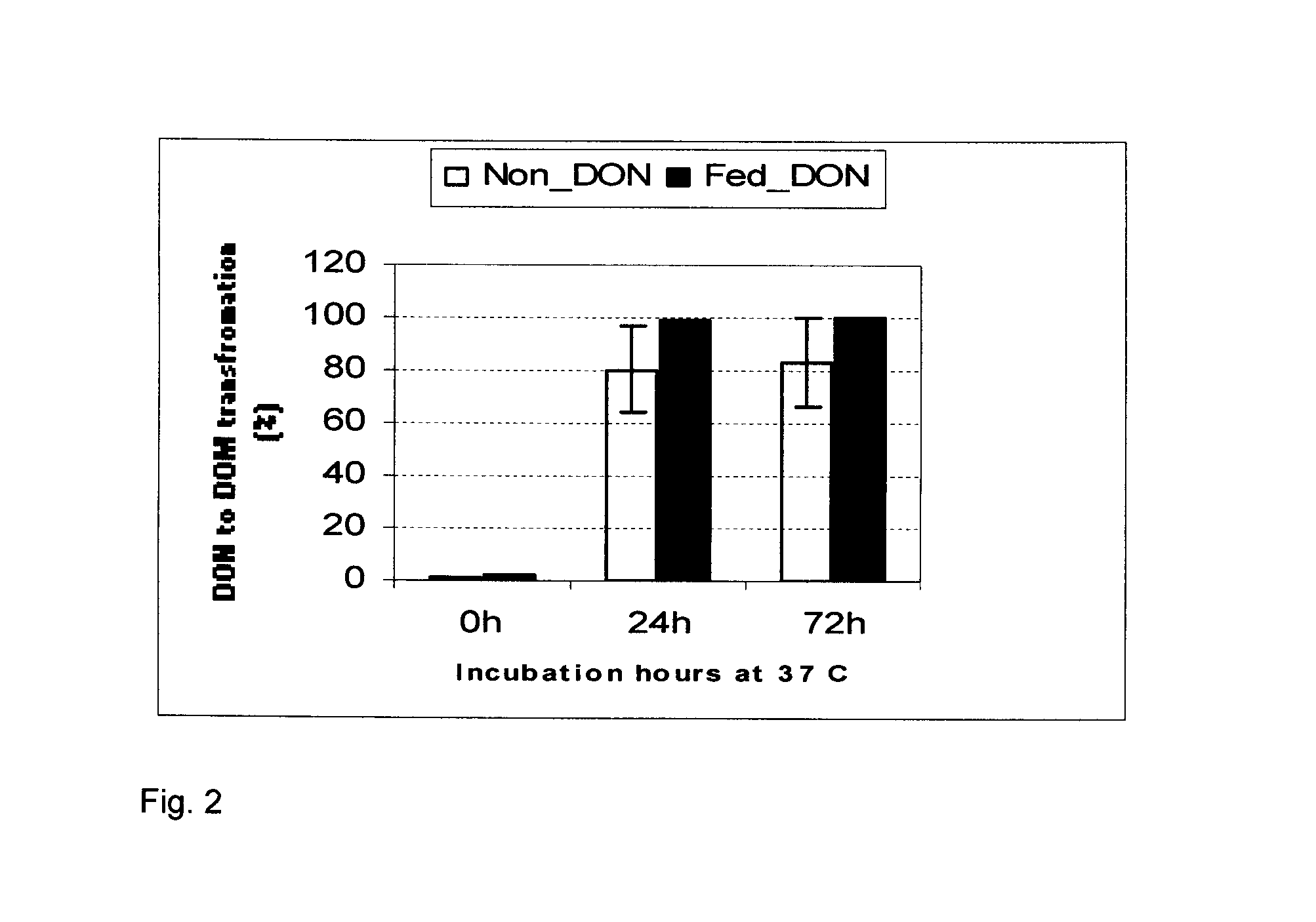
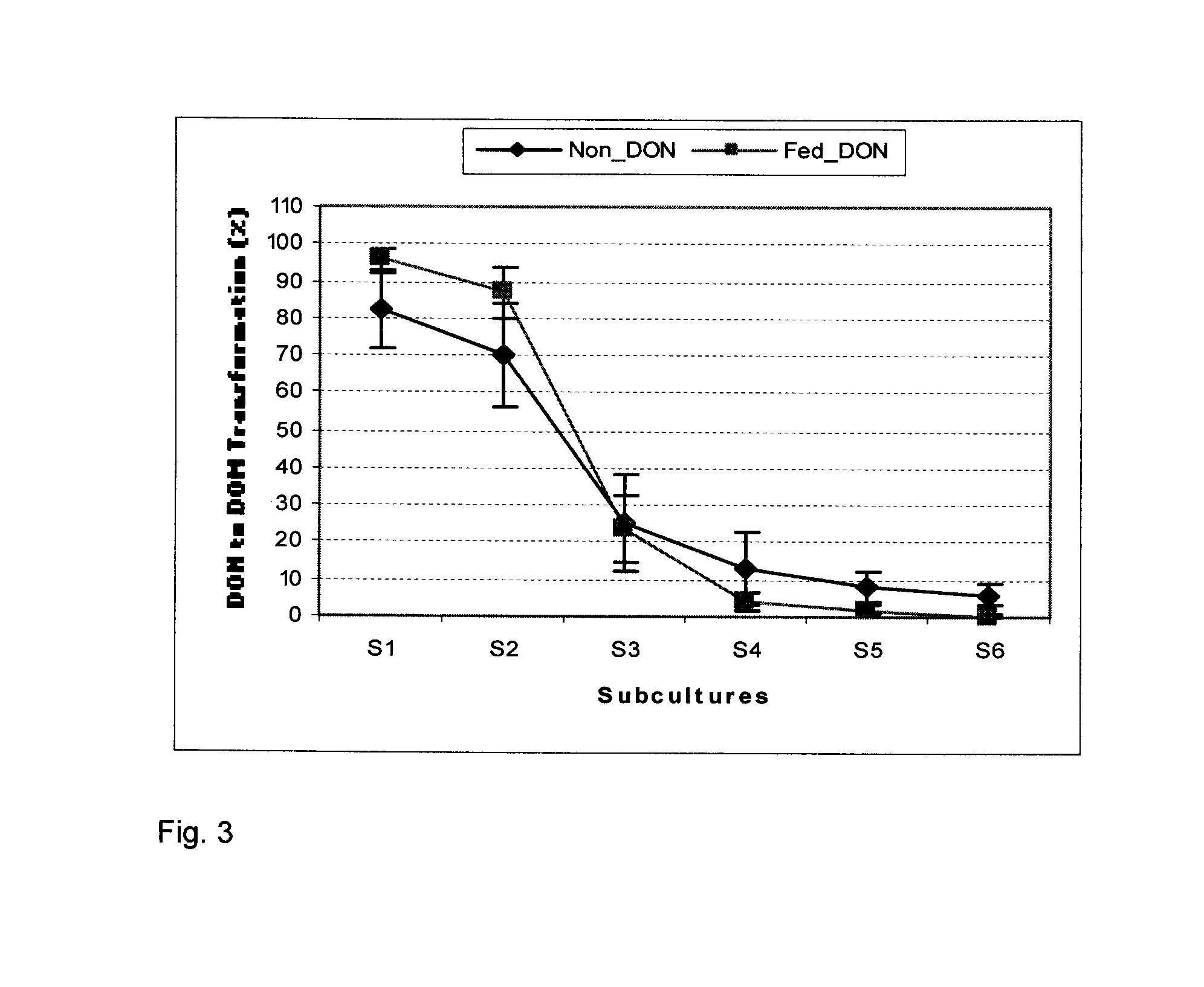
Bacterial isolate, methods of isolating bacterial isolates and methods for detoxification of trichothecene mycotoxins
InactiveUS20120263827A1Preventing and reducing mycotoxin contaminationBiocideMilk preparationBacteroidesTrichothecene
The invention provides a bacterial isolate defined by accession number 040408-1 filed with the International Depository Authority of Canada. The bacteria are capable of detoxifying trichothecene mycotoxins. Also provided are compositions comprising the bacteria and methods of preventing or treating food or foodstuffs that are contaminated or susceptible to contamination with trichothecene mycotoxins. Kits are also provided.
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA
Methods and compositions including spore-forming bacteria for increasing the health of animals
Methods, compositions and bacterial isolates for improving the gastrointestinal health of animals and in particular of poultry are provided herein. The methods include administering an endospore-forming bacteria to an animal. The bacteria are selected for the ability to reduce the growth and presence of bacterial pathogens, such as Salmonella, Clostridium, and Campylobacter, in the gastrointestinal tract of the animal. The bacteria are also selected for the ability to improve at least one production parameter in the animal.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
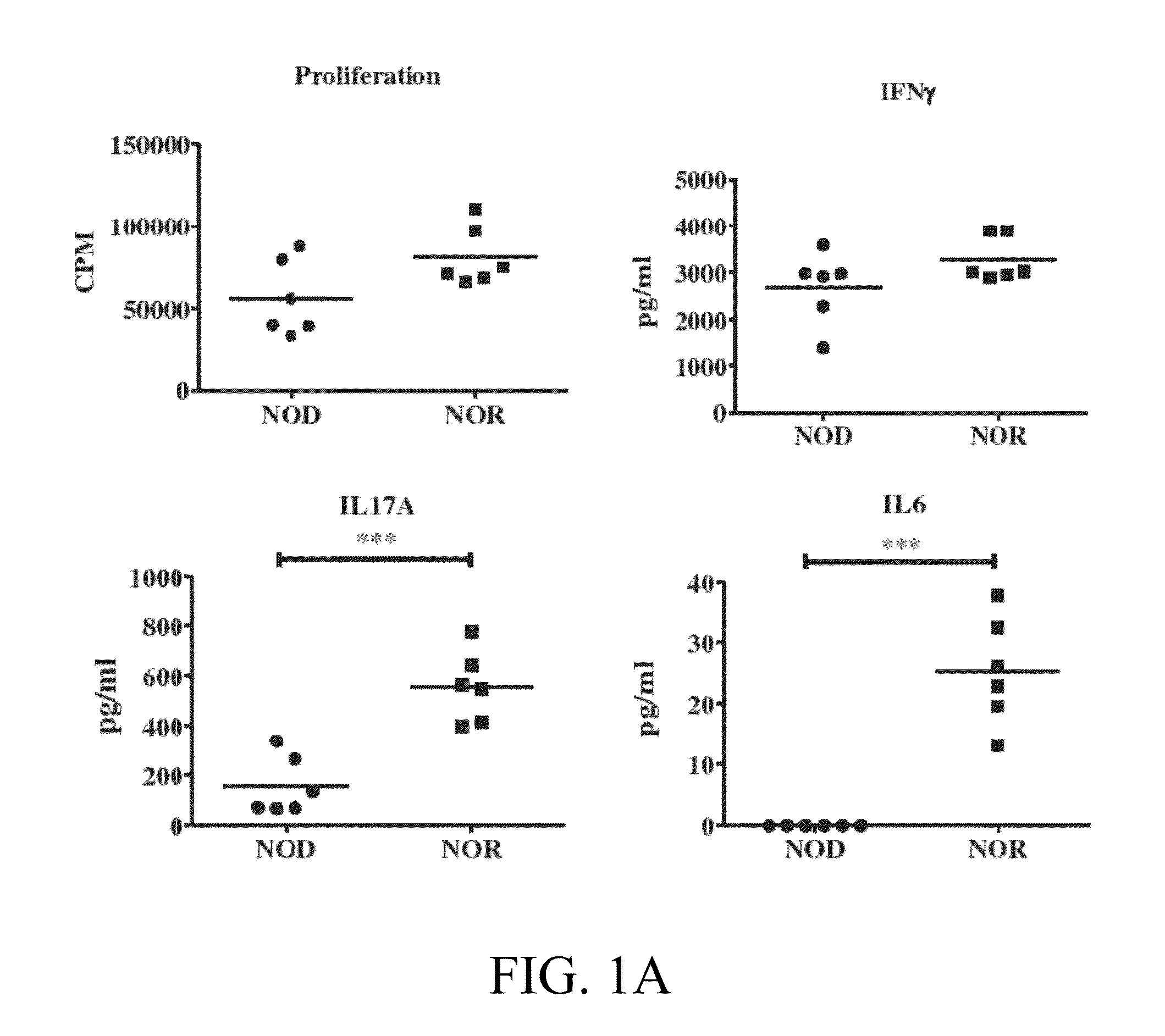
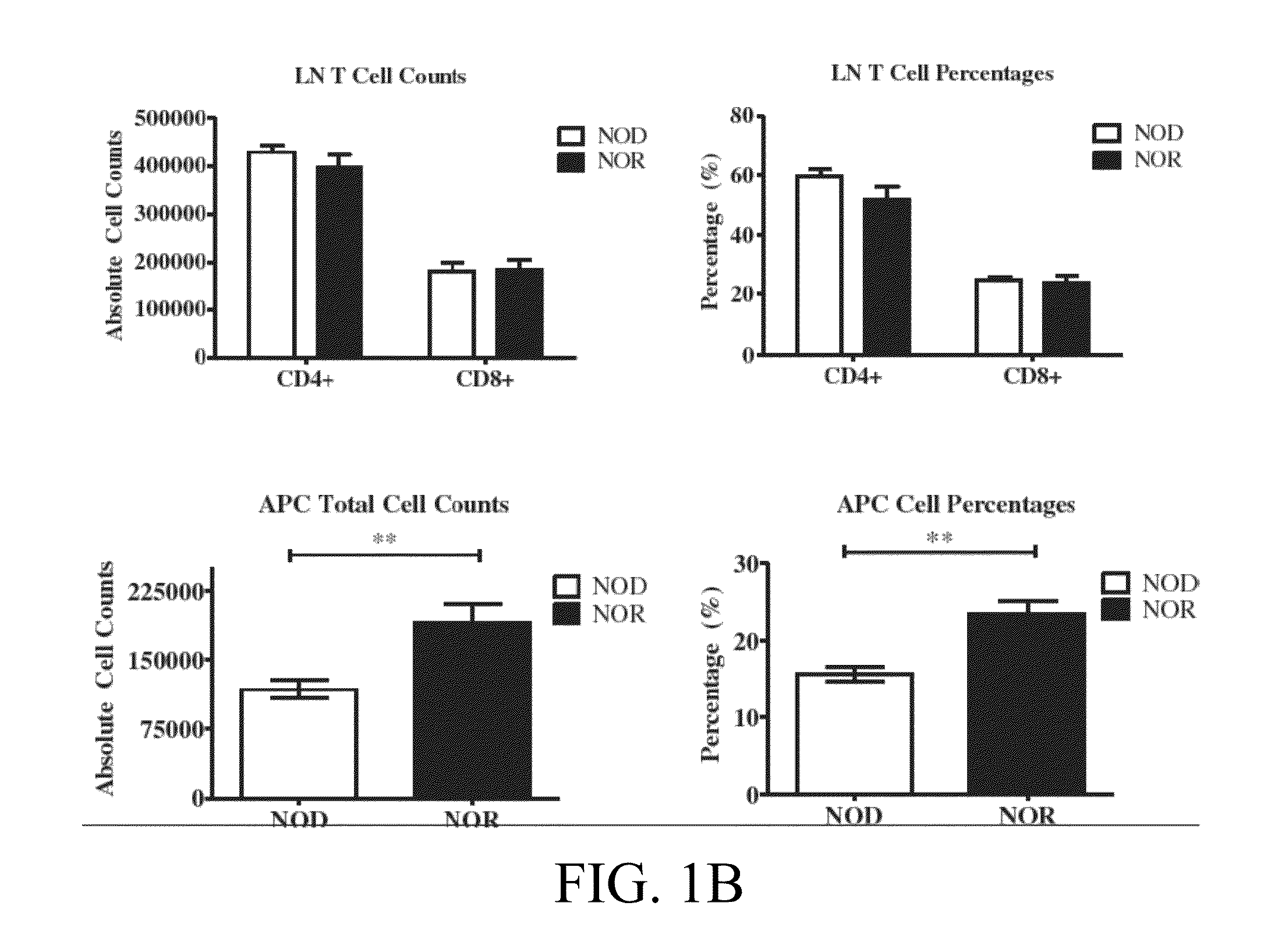
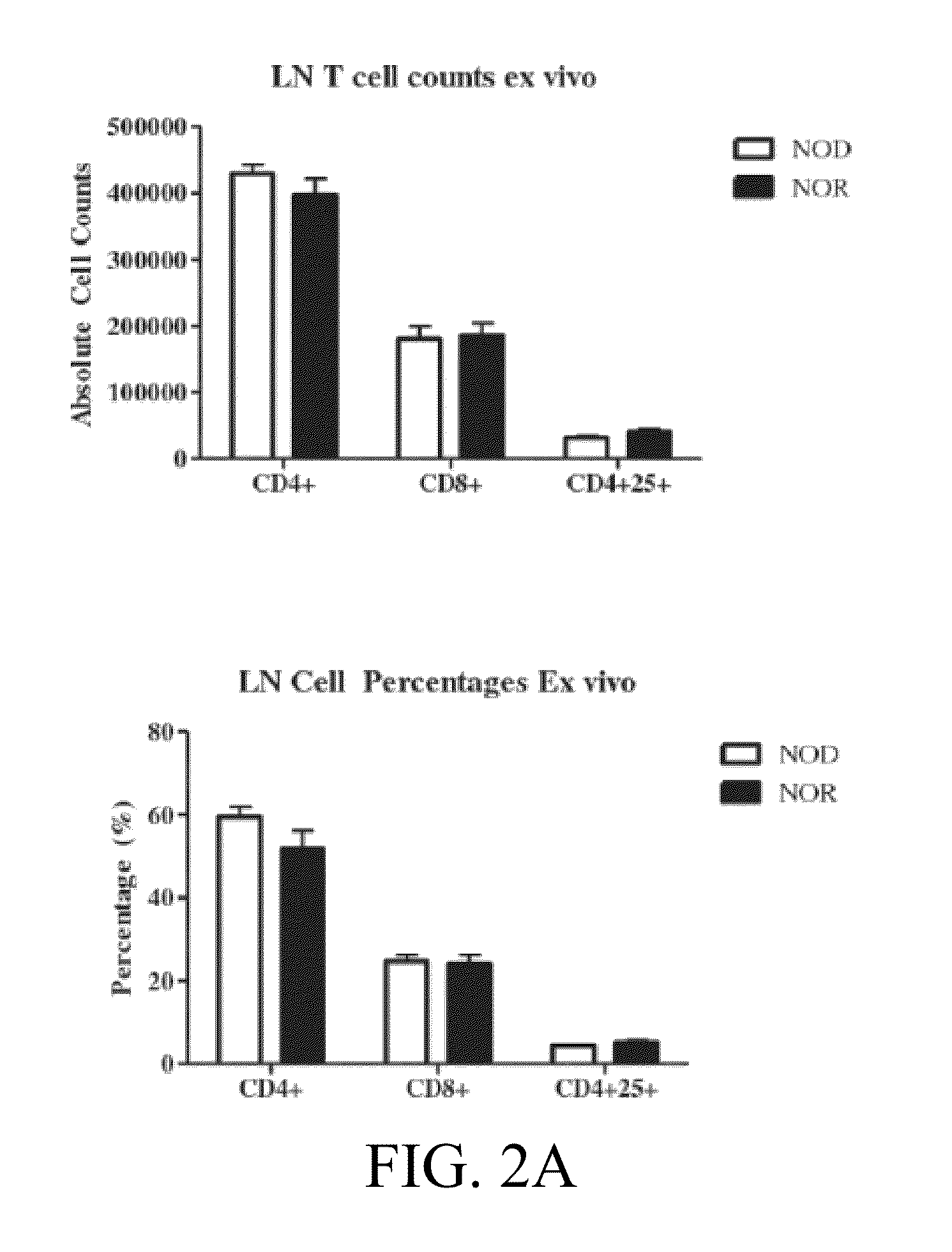
Novel type 1 diabetes vaccines, and methods of use
InactiveUS20140271718A1Prevent and delay onset of and reduce progressionSlow onsetBacterial antigen ingredientsMetabolism disorderClostridiaDendritic cell
The subject invention provides compositions for alleviating type 1 diabetes (T1D). In preferred embodiments, the compositions comprise an effective amount of one or more antigen presenting cells (APCs) that have been pulsed with one or more bacterial isolates and / or compounds from the isolates. The bacteria used to pulse the APCs are, preferably, those that confer upon the APCs the ability to inhibit the generation of diabetes-promoting T cells. In specific embodiments, these bacteria may be, for example, Eubacteria or Clostridia. In a preferred embodiment, the APCs are dendritic cells (DCs).
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Paenibacillus polymyxa schc 33 bacterial strain, and use thereof to combat phytopathogenic fungi in fruits, vegetables or plants
InactiveUS20170303544A1High bactericidal activityProtection attackBiocideBacteriaBacteroidesBiotechnology
Biofungicidal composition from a biologically pure culture of a Chilean bacterial isolate obtained from soils of the seventh region of Maule, Chile, corresponding to Paenibacillus polymyxa SCHC33, strain with the deposit number RGM2141 granted by the depository authority of the Chilean Collection of Microbial Genetic Resources (CChRGM) to be used as an environmentally friendly, biological control agent against fungal plant diseases, particularly fruits susceptible to infection by Botrytis cinerea, efficiently inhibiting conidial germination and mycelium proliferation of said phytopathogenic fungus, furthermore protects plant leaves and fruits from infection by the same fungus, and has the potential to be used in biological control of other fungi and in general of phytopathogenic microorganisms.
Owner:UNIV DE SANTIAGO DE CHILE
Vaccine for periodontal disease
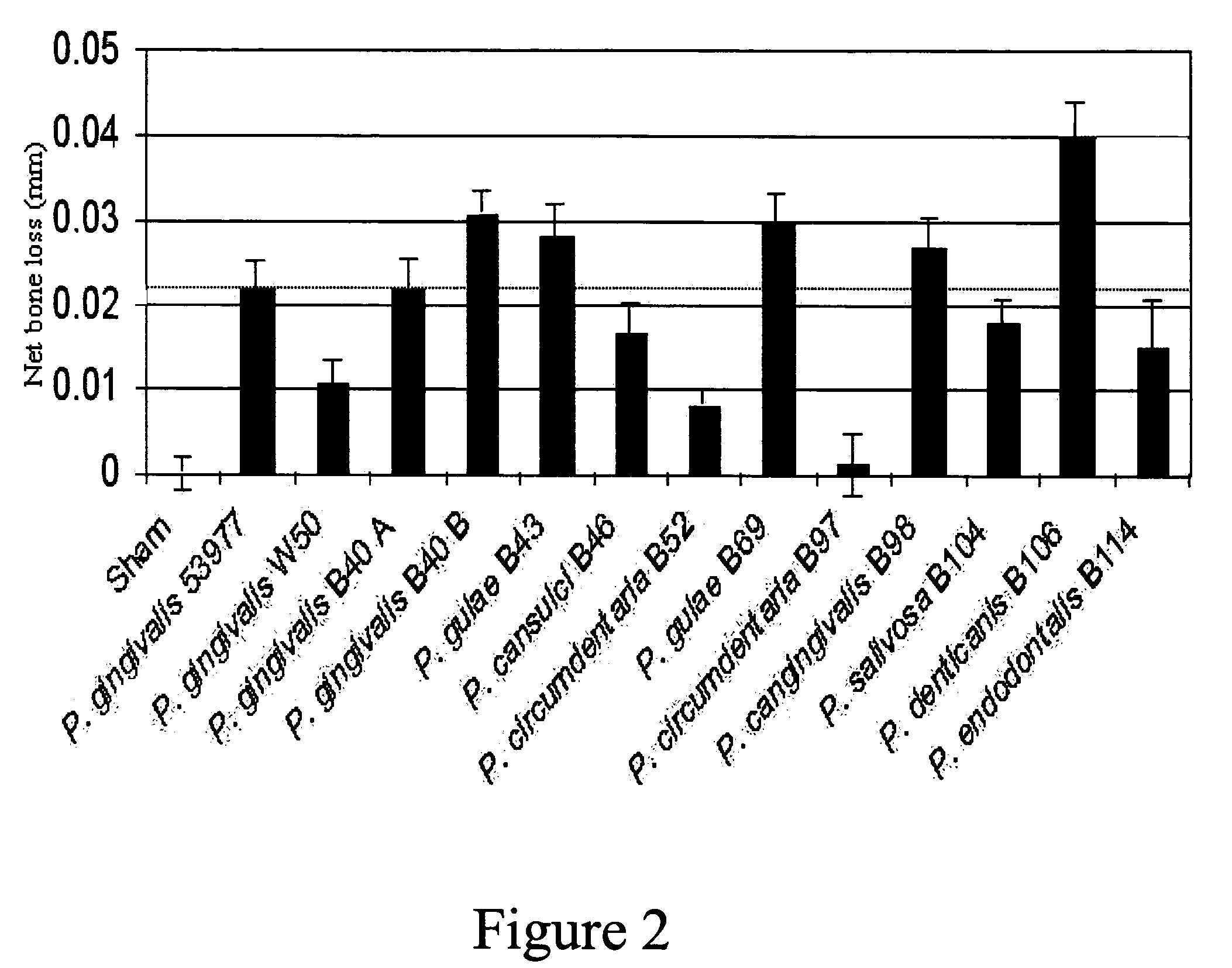
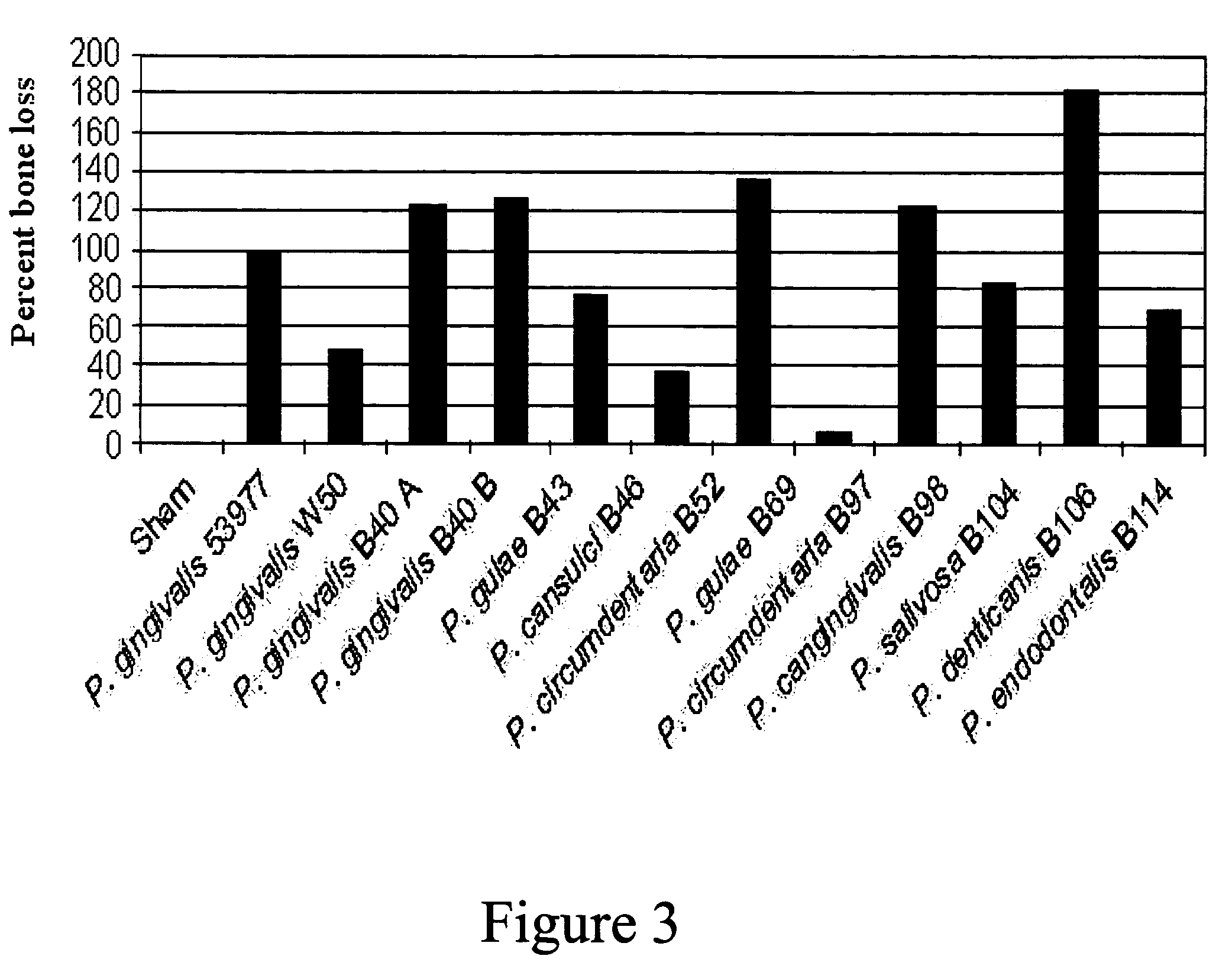
The present invention relates to novel bacterial isolates identified by their 16S rRNA DNA, that cause periodontal disease in companion animals, polynucleotide sequences contained therein, polypeptides encoded by such polynucleotide sequences and vaccines comprising such bacteria, polynucleotides, or polypeptides. Also provided are methods for treating and preventing periodontal disease and kits for detecting and treating periodontal disease kits for detecting and preventing periodontal disease. In addition, methods for assessing the efficacy of a vaccine against periodontal diseases in an animal are provided.
Owner:PFIZER INC +1
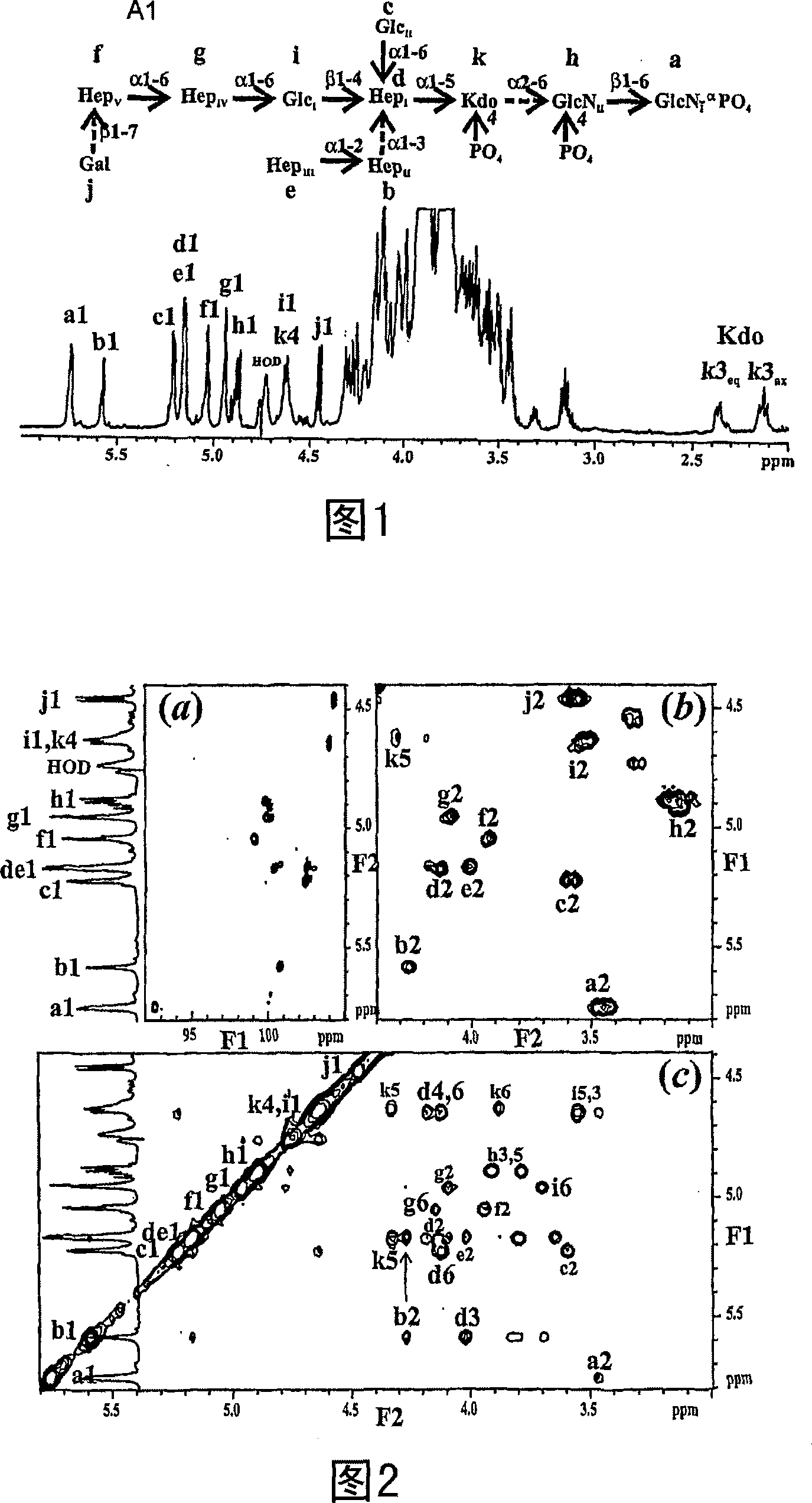
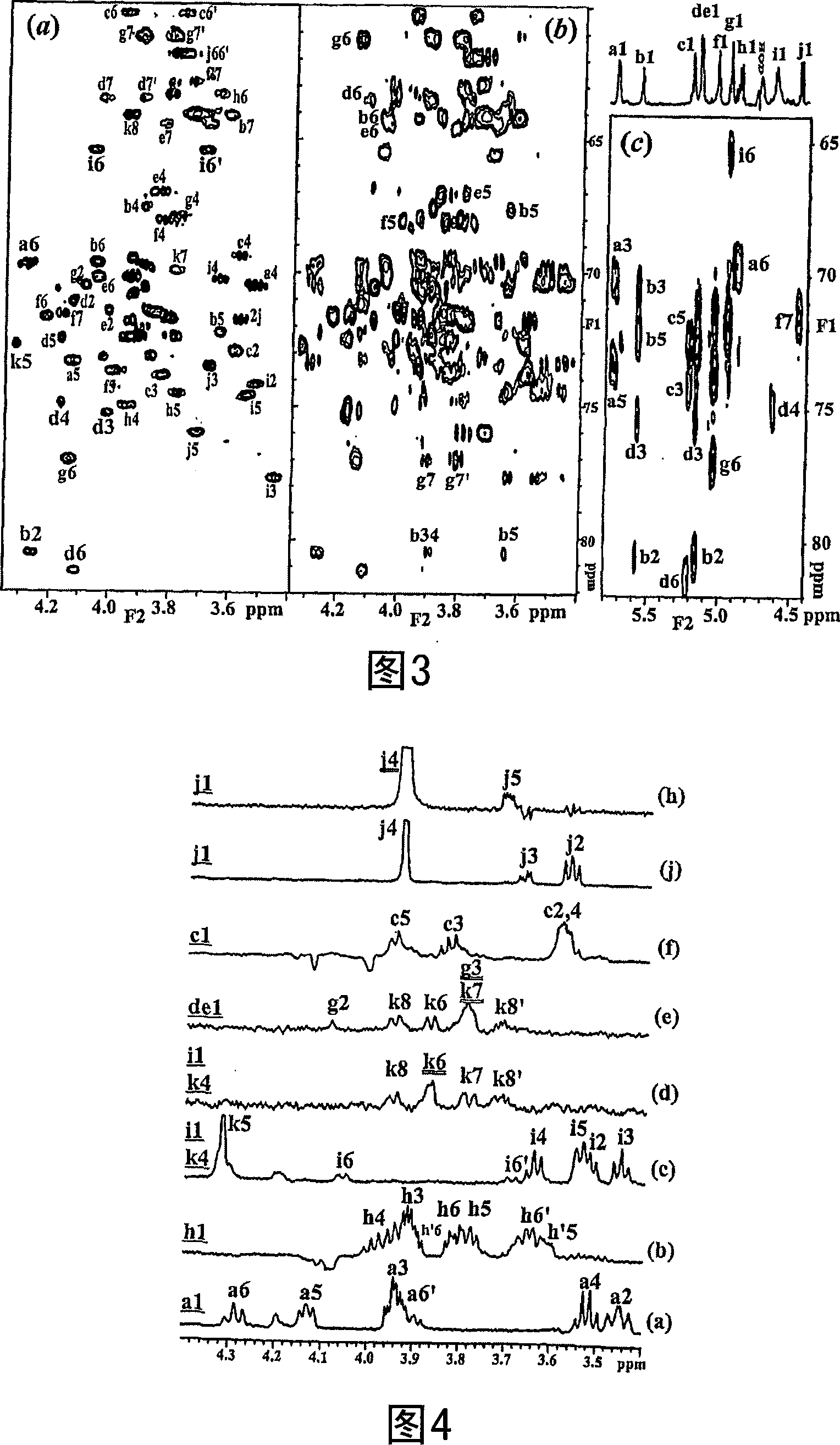
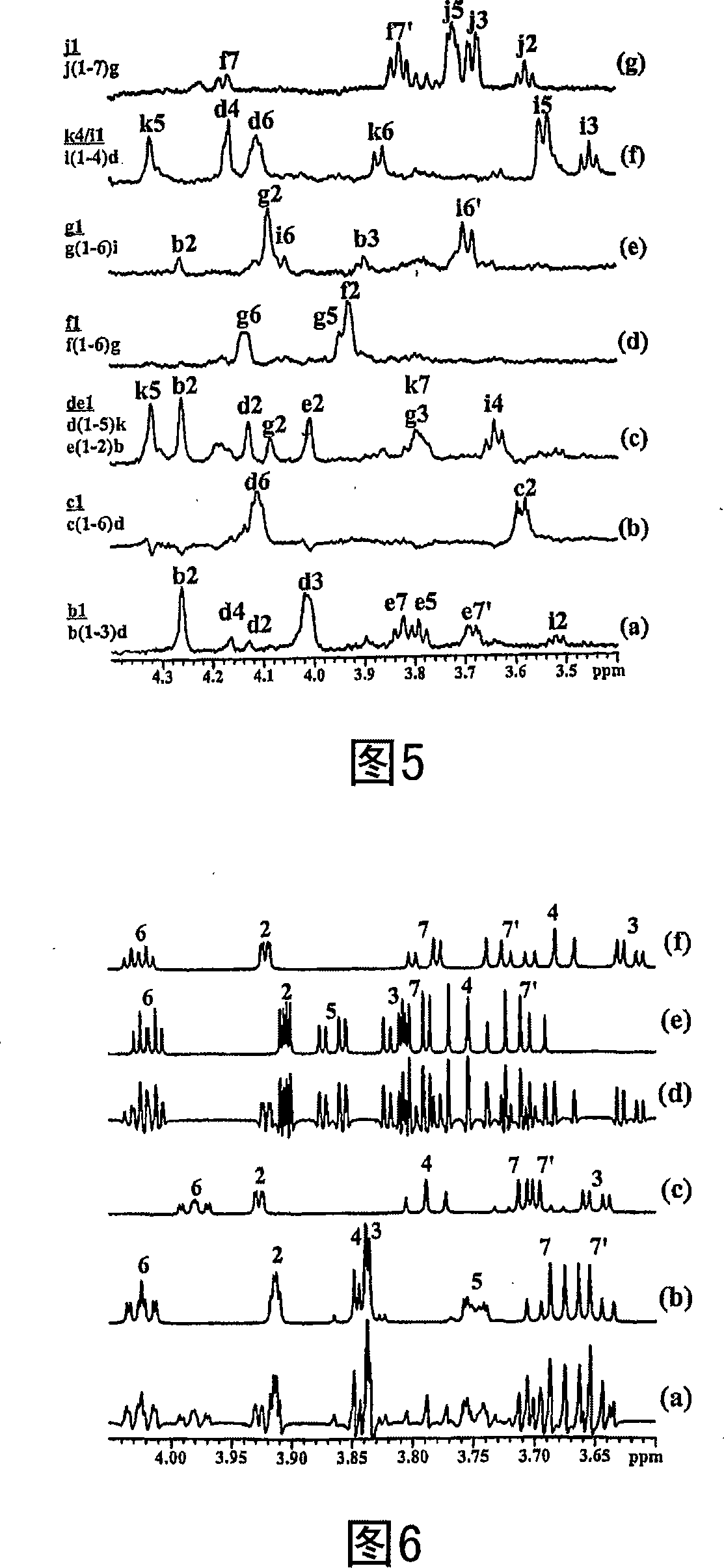
Conserved inner core lipopolysaccharide epitopes as multi-species vaccine candidates
InactiveCN101014698AEsterified saccharide compoundsAntibacterial agentsDiseaseMANNHEIMIA HAEMOLYTICA
A conserved inner-core oligosaccharide epitope expressed on the lipopolysaccharide (LPS) of a range of disease causing pathogenic bacterial isolates, including Actinobacillus pleuropneumoniae (Ap), Mannheimia haemolytica (Mh) and Pasteurella multocida (Pm), is disclosed. Construction of a mutant bacterial strain exclusively expressing the conserved inner core OS epitope as a terminally exposed structure has allowed the identification, production and isolation of an inner core LPS which is common to all three organisms. Further provided are associated vaccines, antibodies raised against the conserved LPS inner core and glycoconjugates comprising the LPS inner core linked to an immunogenic carrier.
Owner:NAT RES COUNCIL OF CANADA
Proteus mirabilis and application thereof in suppression of biofilm and detoxification
ActiveCN102911899AInhibition formationDoes not affect growthAntibacterial agentsBacteriaMetaboliteBULK ACTIVE INGREDIENT
The invention discloses a bacterial isolates relative to suppression of a biofilm and detoxification. The bacterial isolates relative to suppression of the biofilm and detoxification, which is provided by the invention, is especially Proteus mirabilis (Proteus mirabilis)10#, wherein the preservation number of Proteus mirabilis10# in China General Microbiological Culture Collection Center is CGMCC No.6426. The Proteus mirabilis10# CGMCC No.6426, which is provided by the invention, has effects of inhibiting formation of the pathogenic bacteria biofilm and detoxifying. The invention has the following advantages that: (1) through active ingredients (metabolites) of a Proteus mirabilis10# CGMCC No.6426 preparation, growth of the pathogenic bacteria is not influenced, the force is not generated, and the tolerance probability is low; and (2) through active ingredients (metabolites) of a Proteus mirabilis10# CGMCC No.6426 preparation, formation of the pathogenic bacteria biofilm is inhibited and the toxicity of the pathogenic bacteria is reduced.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Process for bio-bleaching of kraft pulp using bacterial consortia
InactiveUS20040011485A1Environment friendly and safe and efficientBacteriaWashing/displacing pulp-treating liquorsBacteroidesMicrobacterium
The present invention relates to an environment friendly, safe, and efficient four-step method of bio-bleaching Kraft pulp using bacterial strains of accession no. MTCC 5096, MTCC 5094, MTCC 5095, and MTCC 5098, a microbial consortium comprising a synergistic mixture of ligninolytic bacterial isolates of accession no. MTCC 5094, MTCC 5095, and MTCC 5098, bacterial strains of accession Nos. MTCC 5096, MTCC 5094, MTCC 5095, and MTCC 5098, and a process of preparing an inoculum of the bacterial isolate of accession no. MTCC 5096, further, a process for the preparation of a consortium comprising the ligninolytic bacterial isolates of accession nos. MTCC 5094, MTCC 5095, and MTCC 5098, in addition, a process for the preparation of pulp suspension for the bio-bleaching.
Owner:COUNCIL OF SCI & IND RES
Microbial biodegradation of phosphonates
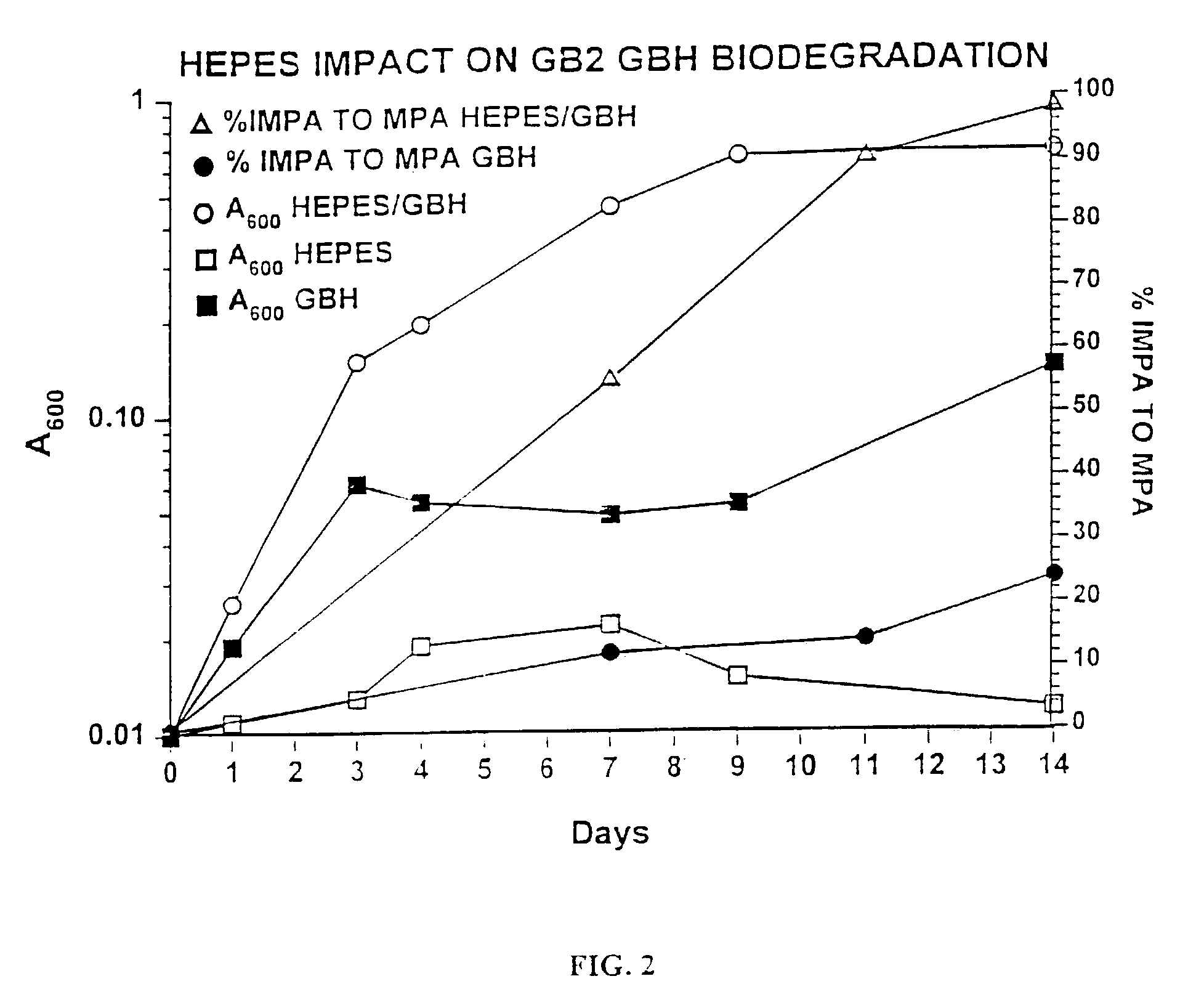
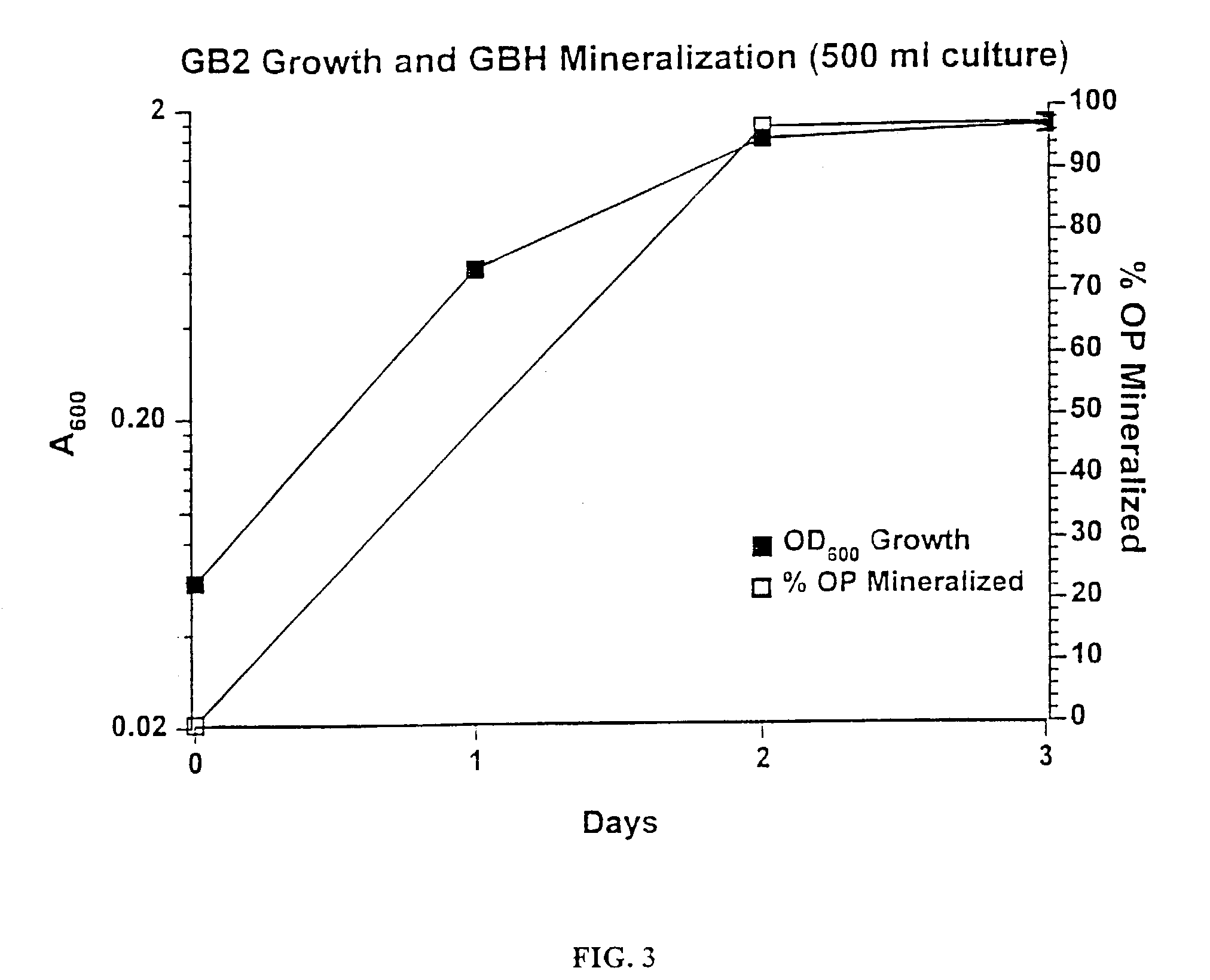
A biodegradation process for the organophosphonate product of Sarin (O-isopropyl methylphosphonofluoridate) hydrolysis, i.e., isopropylmethylphosphonate (IMPA). This process provides a feasible biodegradation demilitarization alternative to Sarin incineration. Public opposition of nerve agent incineration is widespread, and alternative methods are sought to help the U.S. Army meet the 2007 demilitarization deadline imposed by the Chemical Weapons Convention. This process uses a two-step approach to IMPA biodegradation. In the first step, a concentrated IMPA solution is used as the sole nutritional carbon and phosphorus source for microbial cultures. The second step involves diluting the culture and adding an inexpensive carbon source to encourage bacterial phosphate assimilation. The biodegradation typically involves a consortium of microorganisms comprising Methylobacterium radiotolerans GB21, Agrobacterium tumefaciens GB2GA, Klebsiella oxytoca GB2CS, GB272, Aureobacterium sp. GB2 and three bacterial isolates belonging to the same species GB23, GB272, and GB292.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
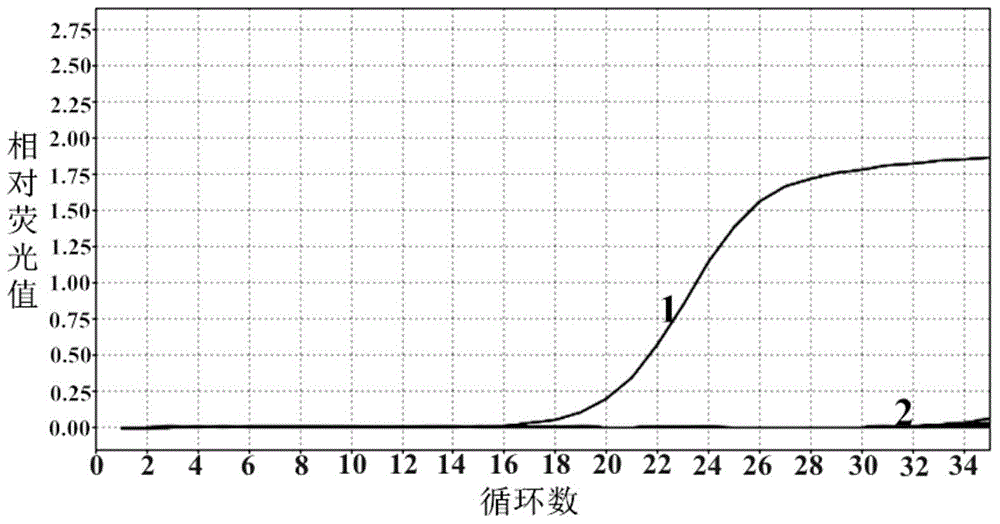
Bacterial isolate and methods for detoxification of trichothecene mycotoxins
ActiveUS8642317B2Preventing and reducing mycotoxin contaminationMilk preparationBiocideBacteroidesTrichothecene
The invention provides a bacterial isolate defined by accession number 180507-1 filed with the International Depository Authority of Canada. The bacteria are capable of degrading trichothecene mycotoxins. Also provided are compositions comprising the bacteria and methods of preventing or treating food, foodstuffs, crops and harvested crops that are contaminated or susceptible to contamination with trichothecene mycotoxins. Kits are also provided.
Owner:AGRI & AGRI FOOD
Bacterial isolate and methods for detoxification of trichothecene mycotoxins
ActiveUS20100239537A1Preventing and reducing mycotoxin contaminationMilk preparationBiocideBacteroidesTrichothecene
The invention provides a bacterial isolate defined by accession number 180507-1 filed with the International Depository Authority of Canada. The bacteria are capable of degrading trichothecene mycotoxins. Also provided are compositions comprising the bacteria and methods of preventing or treating food, foodstuffs, crops and harvested crops that are contaminated or susceptible to contamination with trichothecene mycotoxins. Kits are also provided.
Owner:AGRI & AGRI FOOD
Biofungicidal composition for controlling phytopathogenic fungi
Biofungicide composition derived from a biologically pure culture of a Chilean bacterial isolate obtained from the skin of grapes, corresponding to Serratia plymuthica CCGG2742, to be used as an environmentally friendly biological control agent against fungal diseases of vegetables, in particular fruits susceptible to the infection of Botrytis cinerea, efficiently preventing the germination of conidia and the proliferation of mycelia of said phytopathogenic fungus, furthermore protecting the plant's leaves and fruits from the infection by the same fungus, and having the potential of being used in the biological control of other phytopathogenic fungus and microorganisms.
Owner:UNIV DE SANTIAGO DE CHILE
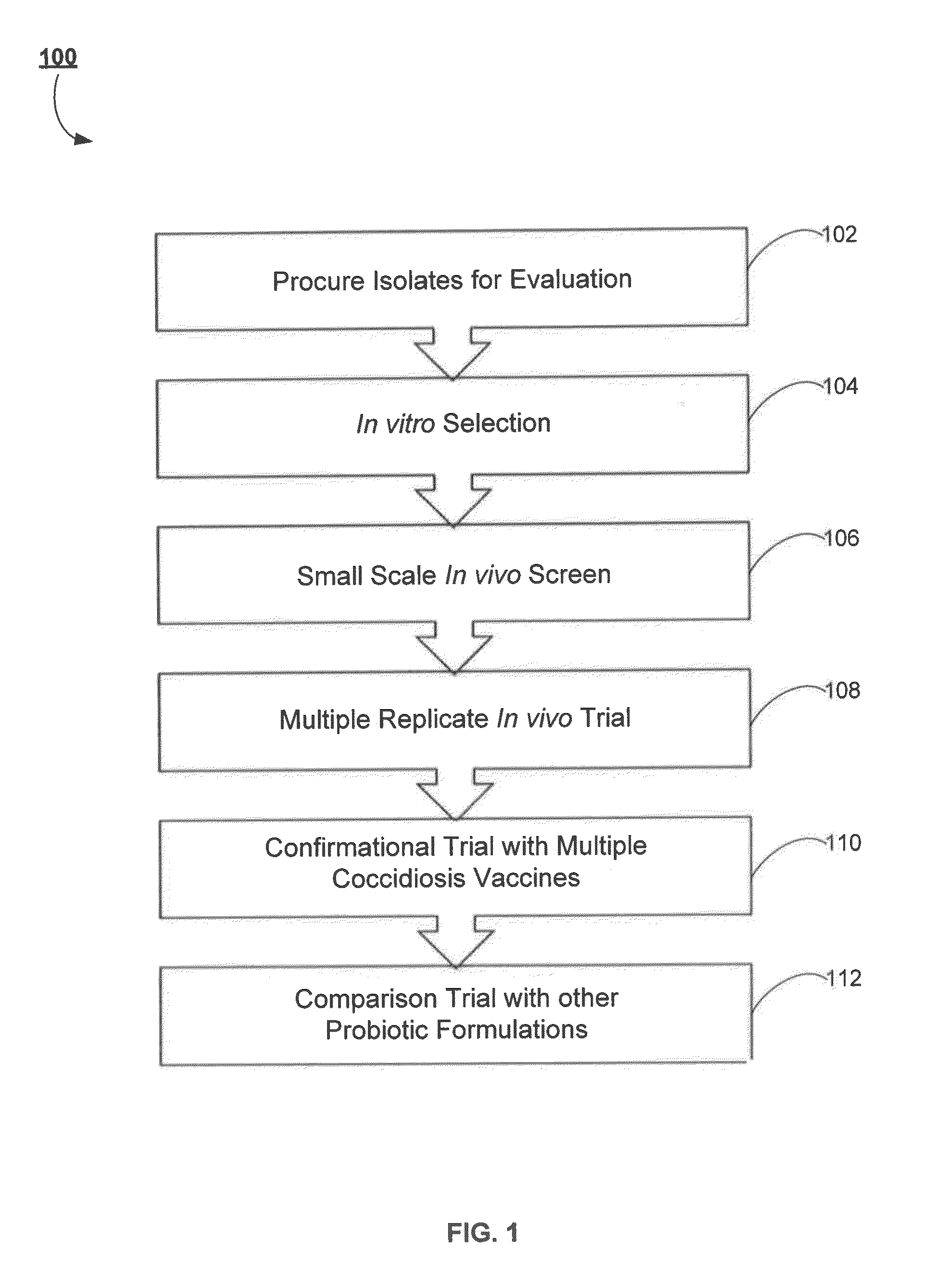
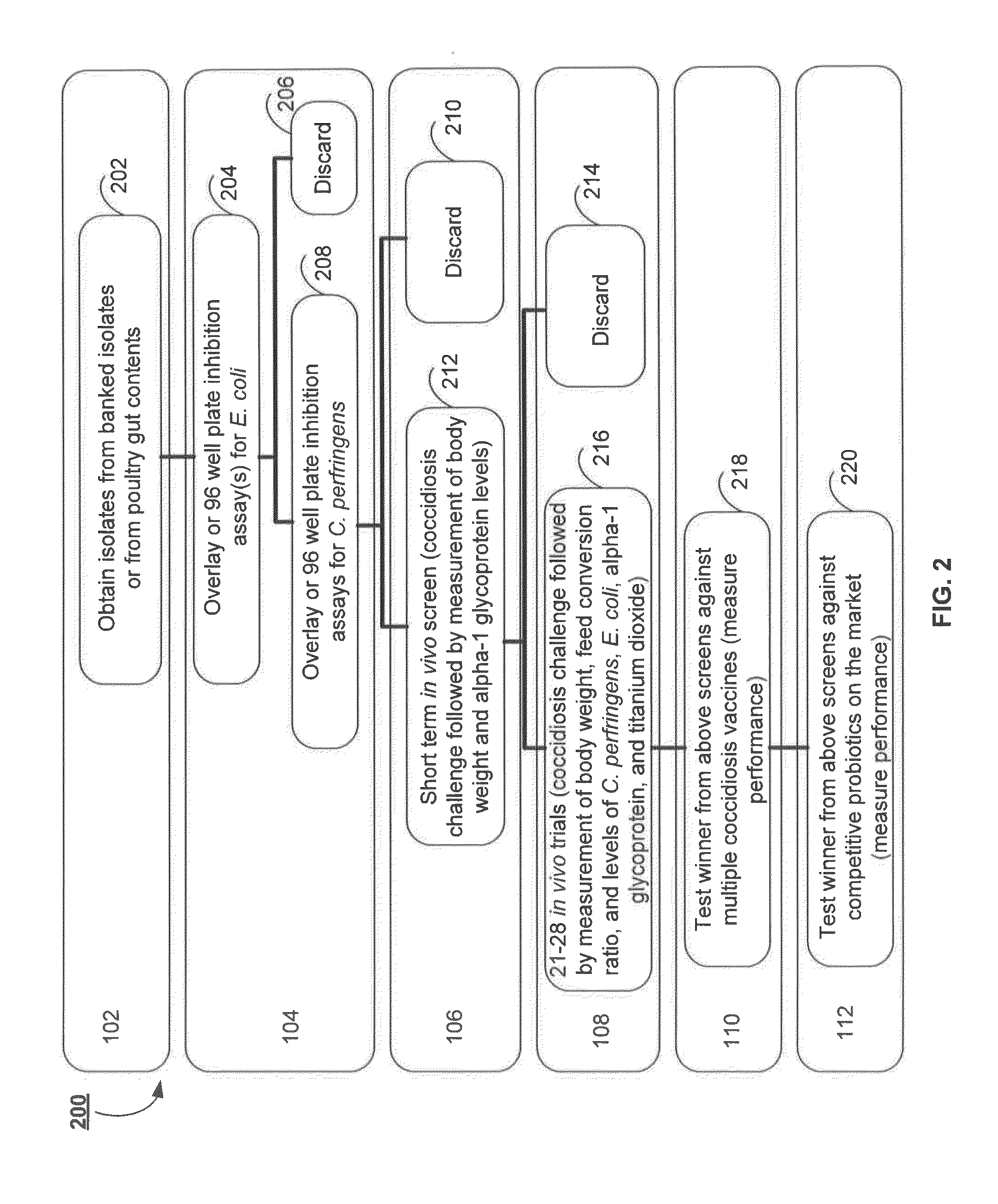
Probiotic for amelioration of coccidiosis vaccine reaction
ActiveUS20150182564A1Reduce inflammationReduce severityBacteria material medical ingredientsMicrobiological testing/measurementBiotechnologyAnimal science
Methods for improving the health of agricultural poultry are provided. For example, methods including selecting specific bacteria to form a probiotic for the administration with coccidiosis vaccines for the reduction of adverse effects associated with the coccidiosis vaccines are disclosed. In accordance with various aspects of the present disclosure, a bacterial isolate, probiotic and or treatment may be obtained by novel screening methods resulting in products that are advantageously administered with or about the same time as a coccidiosis vaccine.
Owner:NOVOZYMES AS
Intracellular proteinacious antimicrobial agents from lactic acid bacteria derived from fermented food samples
The present invention is directed bacterial isolates that produce proteinacious antimicrobial agents effective against a variety of food borne pathogens. The invention includes the bacteria, bacteriocin preparations derived from the bacteria and methods by which the preparations may be used.
Owner:KRAFT FOODS GRP BRANDS LLC +1
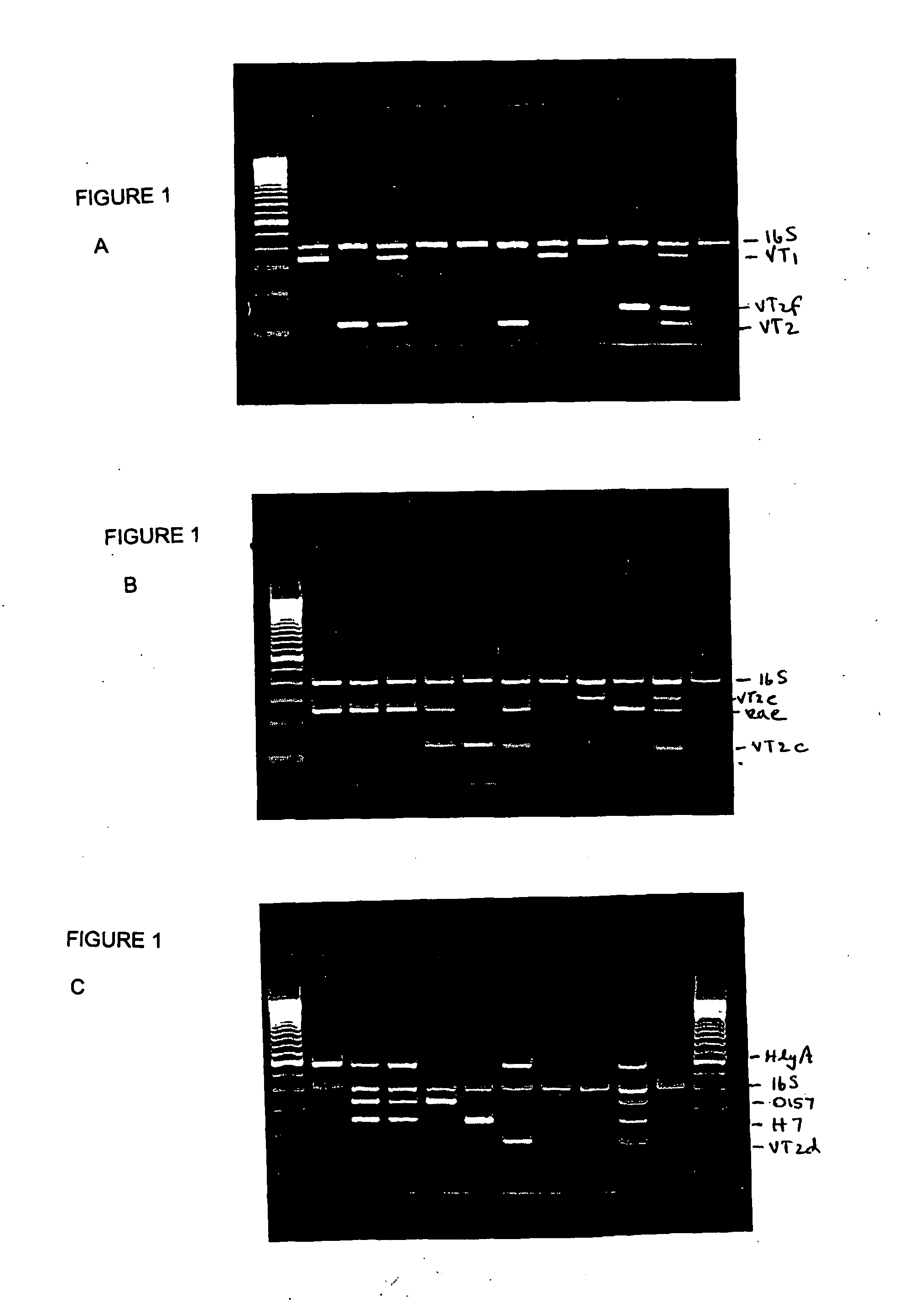
Major virulence factor detection and verocytontoxin type 2 subtype from clinical e. coli isolates using a one-step multiplex pcr
InactiveUS20060051751A1Sugar derivativesMicrobiological testing/measurementEscherichia coliVirulent characteristics
A single kit comprising 3 multiplex PCR assays that can detect in E. coli the presence of the 8 virulence genes: eaeA, EHEC-HlyA, Stx1 (VT1), Stx2 (VT2), Stx2c (VT2c), Stx2d (VT2d), Stx2e (VT2e) and Stx2f (VT2f) is described. In addition, the kit can detect the two critical serotypes (O157 and H7) and identify the species (Escherichia coli) simultaneously using a one step reaction. Following evaluation in our hands, this PCR kit has been used to detect the above 11 components of disease-causing E. coli in a fast, accurate, reliable and specific fashion. These kits can be used on bacterial isolates and has the potential for use directly on foods and environmental samples.
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA REPRESENTED BY THE MIN OF HEALTH
Culture method for improving yield and viscosity of bacterial polysaccharides
InactiveCN103305566AIncrease productionHigh viscosityMicroorganism based processesFermentationAmpicillinStress factor
The invention provides a culture method for improving yield and viscosity of bacterial polysaccharides. By using the characteristic that penbritin can be simultaneously used as a mutagenic agent and bacterial cell wall peptidoglycan to synthesize stress factors, the penbritin is added to subsequent subculture processes of bacterial isolates for producing different polysaccharides; the adding amount of the penbritin is gradually increased along with an increase of passage number, so as to improve thrill on bacterial cells; and a series of high-producing strains with different viscosities and improvement degrees can be screened in the continuous passage process, so as to adapt to different application requirements. Therefore, the production cost is reduced.
Owner:ZHEJIANG UNIV
Culture medium for screening bacteria with siderophilic capacity and phosphate-solubilizing capacity simultaneously
InactiveCN104357537AImprove separation and screening efficiencyShorten screening timeMicrobiological testing/measurementPhosphateChrome azurol S
The invention discloses a culture medium for screening bacteria with siderophilic capacity and phosphate-solubilizing capacity simultaneously. The formula for preparing 100 mL of the culture medium contains the following components: 3-8 mL of CAS (Chrome Azurol S) blue detection liquid, 0.1-0.3 mL of 1 mmol / L CaCl2 solution, 0.1-0.3 mL of 1 mmol / L MgSO4.7H2O solution, 4-8 mL of acid hydrolyzed casein solution with the mass concentration of 8-12%, 0.3-0.8 g of Ca3(PO4)2 and 1-3 g of agar powder. The invention further discloses a preparation method of the culture medium. Through the adoption of the culture medium disclosed by the invention, bacterial isolates with siderophilic capacity and phosphate-solubilizing capacity simultaneously can be quickly obtained, so that the screening or detection time is shortened, and the screening efficiency is improved.
Owner:SHANDONG INST OF POMOLOGY
Bacteriocin and new bacterial isolates
New bacteriocin and / or new lactic acid-producing strain are used for at least reducing the planting level of at least one target bacteria in animals, particularly in domestic fowl.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF AGRI (US) +1
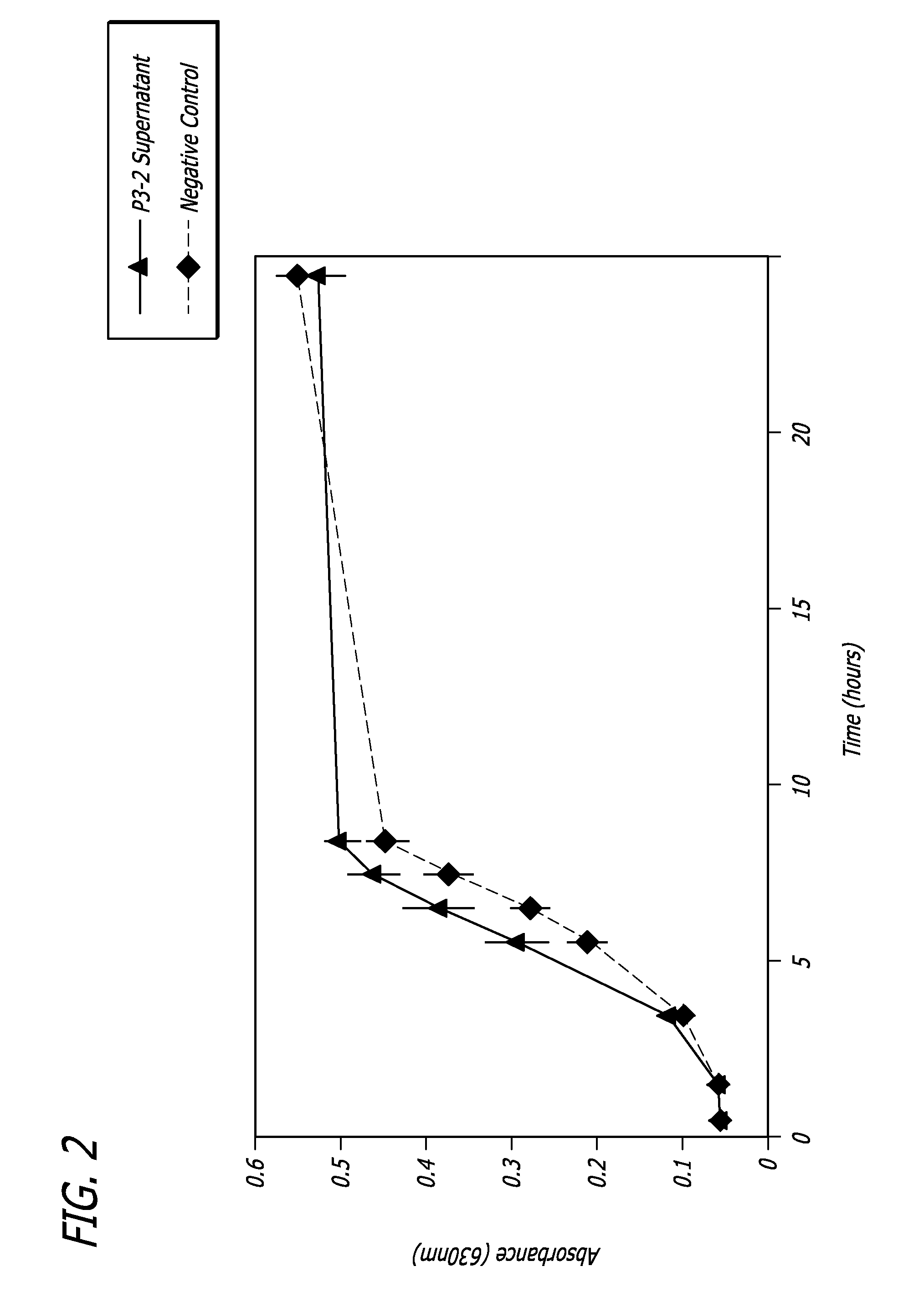
Marine bacterial substances, medical devices, and methods for biofilm inhibition
InactiveUS20120201869A1Inhibit growthInhibition formationAntibacterial agentsCosmetic preparationsBiofilm growthMicrobiology
Disclosed herein are marine bacterial substances, methods, and medical devices that inhibit biofilm growth and / or formation. Substances of the present disclosure are products or byproducts of P3-2 (ATCC PTA-6763), P4-4 (ATCC PTA-6682), P5-2 (ATCC PTA-6764), or P6-6 (ATCC PTA-6766) marine bacterial isolates.
Owner:BURZELL CYNTHIA K
Conserved Inner Core Lipopolysaccharide Epitopes as Multi-Species Vaccine Candidates
InactiveUS20080008723A1Poorly immunogenicImproving immunogenicityAntibacterial agentsCosmetic preparationsBacteroidesDisease
A conserved inner-core oligosaccharide epitope expressed on the lipopolysaccharide (LPS) of a range of disease causing pathogenic bacterial isolates, including Actinobacillus pleuropneumoniae (Ap), Mannheimia haemolytica (Mh) and Pasteurella multocida (Pm), is disclosed. Construction of a mutant bacterial strain exclusively expressing the conserved inner core OS epitope as a terminally exposed structure has allowed the identification, production and isolation of an inner core LPS which is common to all three organisms. Further provided are associated vaccines, antibodies raised against the conserved LPS inner core and glycoconjugates comprising the LPS inner core linked to an immunogenic carrier.
Owner:NAT RES COUNCIL OF CANADA
Conserved inner core lipopolysaccharide epitopes as multi-species vaccine candidates
InactiveUS7759070B2Wide coveragePreventing human diseaseAntibacterial agentsOrganic active ingredientsDiseaseMANNHEIMIA HAEMOLYTICA
A conserved inner-core oligosaccharide epitope expressed on the lipopolysaccharide (LPS) of a range of disease causing pathogenic bacterial isolates, including Actinobacillus pleuropneumoniae (Ap), Mannheimia haemolytica (Mh) and Pasteurella multocida (Pm), is disclosed. Construction of a mutant bacterial strain exclusively expressing the conserved inner core OS epitope as a terminally exposed structure has allowed the identification, production and isolation of an inner core LPS which is common to all three organisms. Further provided are associated vaccines, antibodies raised against the conserved LPS inner core and glycoconjugates comprising the LPS inner core linked to an immunogenic carrier.
Owner:NAT RES COUNCIL OF CANADA
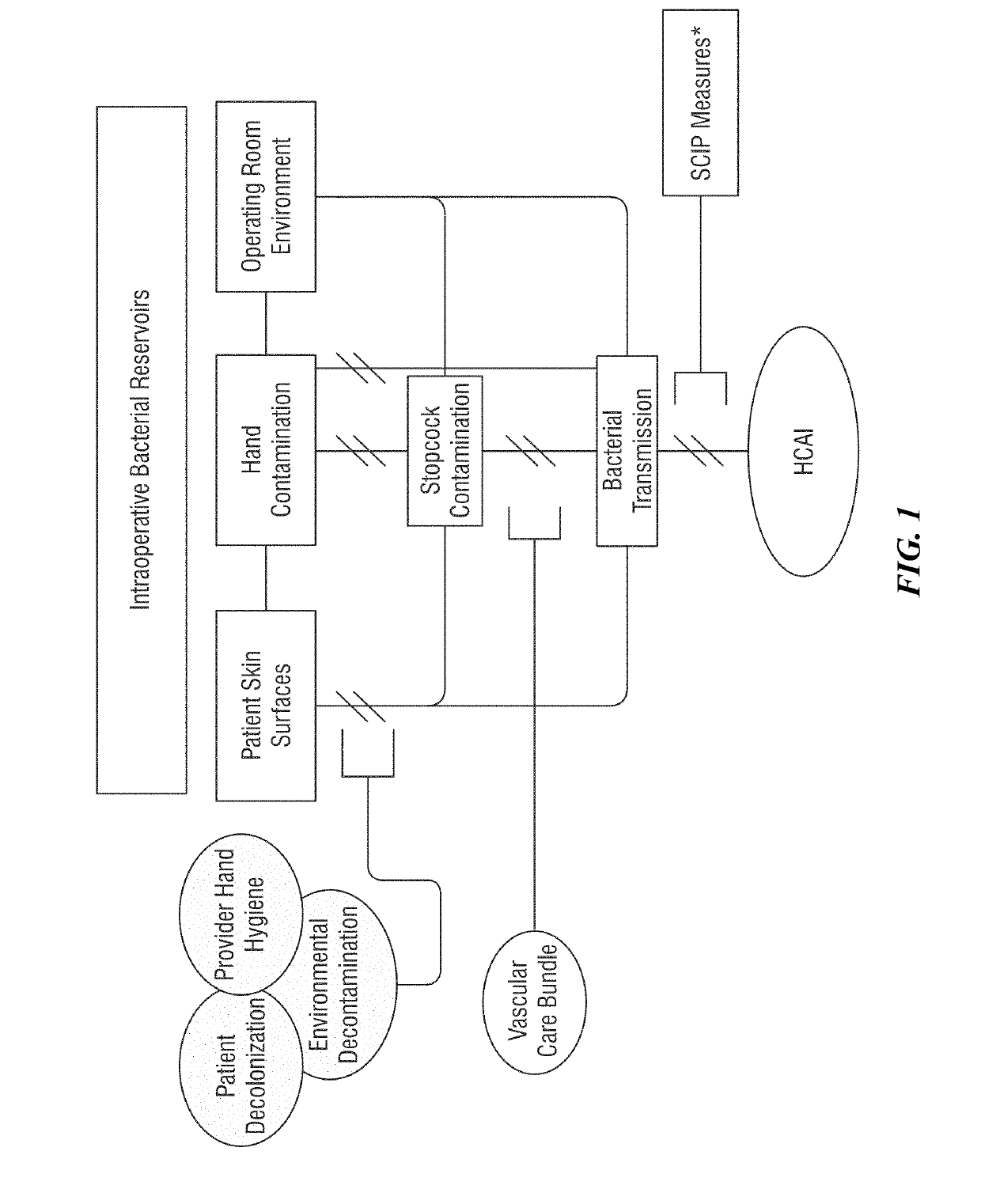
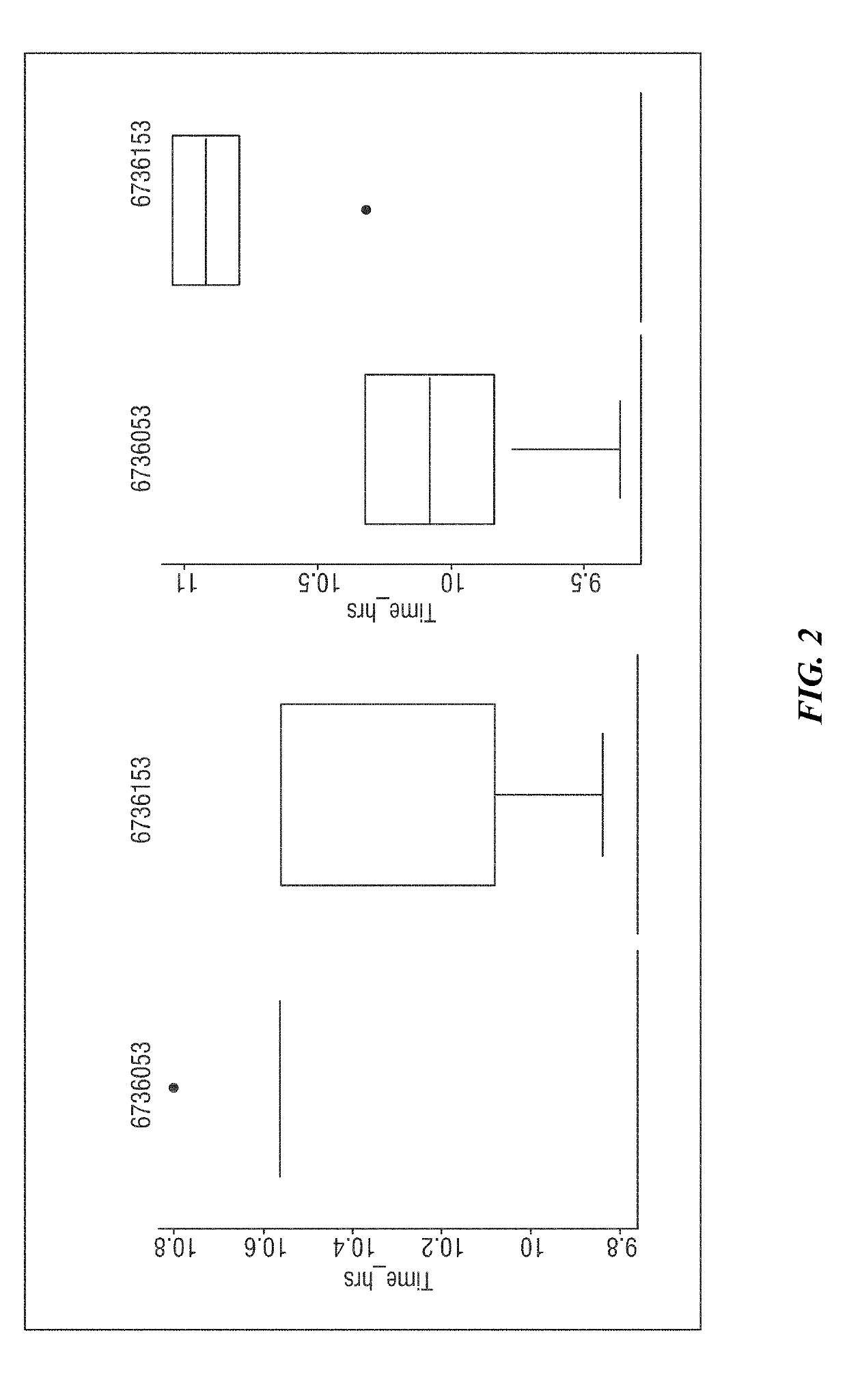
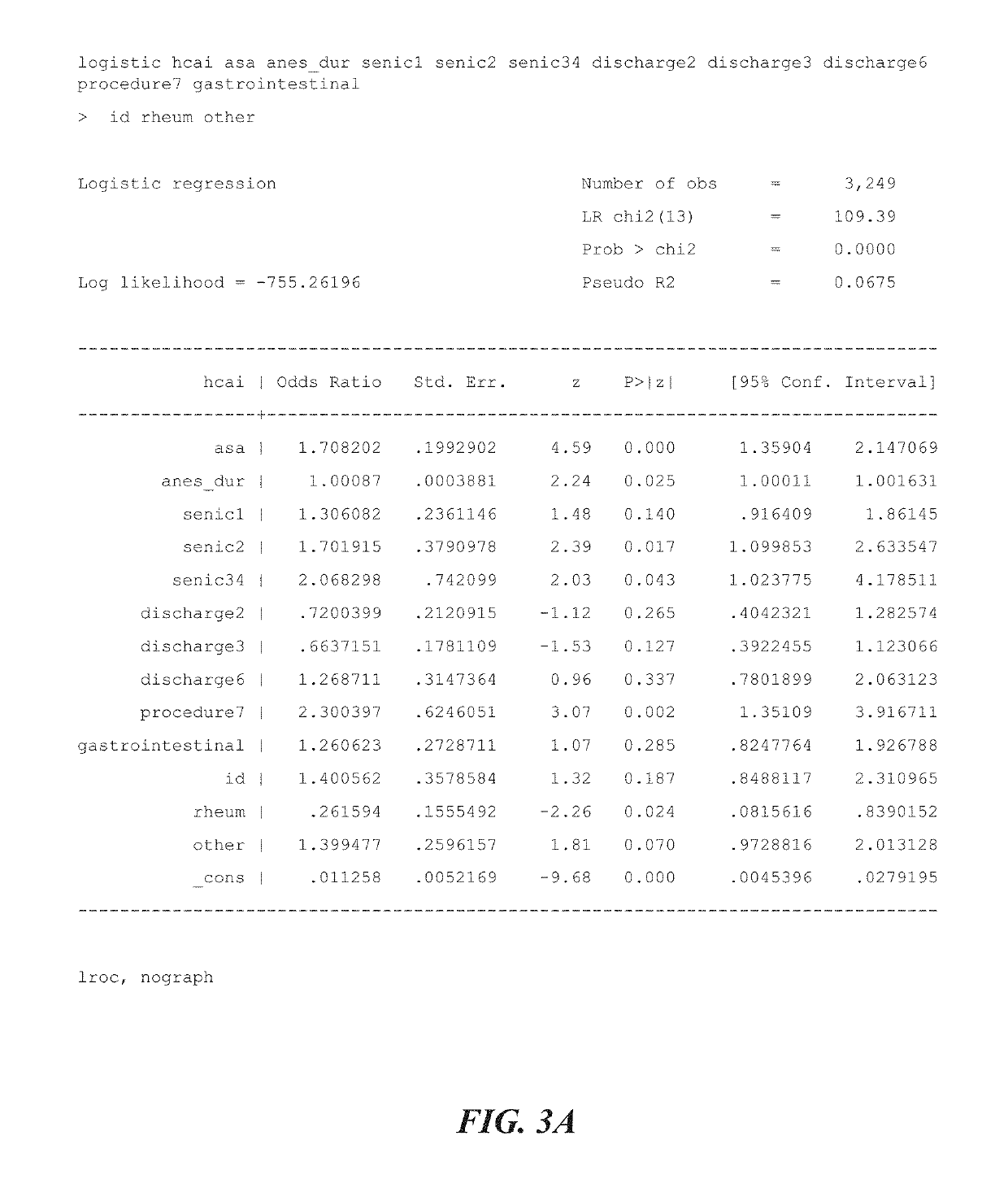
Multi-level, laboratory-based surveillance system for detection of intraoperative "eskape" bacterial pathogens for hcai prevention
PendingUS20190226004A1Medical data miningDigital data processing detailsComputer scienceBacterial isolate
The present invention provides systems and methods for surveillance, diagnosis, and evaluation of high risk bacterial transmission events. The systems and methods utilize software and computational systems that automate identification, surveillance, and communication. The invention further includes archival systems for use in the systems and methods that compile bacterial isolates linked to information about patients, pre-operative, intra-operative, or post-operative arenas, healthcare providers, and the like.
Owner:RDB BIOINFORMATICS LLC
Marine bacterial substances, medical devices, and methods for biofilm inhibition
Disclosed herein are marine bacterial substances, methods, and medical devices that inhibit biofilm growth and / or formation. Substances of the present disclosure are products or byproducts of P3-2 (ATCC PTA-6763), P4-4 (ATCC PTA-6682), P5-2 (ATCC PTA-6764), or P6-6 (ATCC PTA-6766) marine bacterial isolates.
Owner:BURZELL CYNTHIA K
Methods and products for treating gastrointestinal disorders
Described herein are compositions and methods for delivering microbial therapeutic agents useful for the treatment of conditions associated with dysbacteriosis of intestinal flora. In various aspects, the present invention provides compositions and methods useful for treating or preventing inflammatory bowel disease (IBD) in a subject in need thereof. For example, the present invention relates, in part, to a plurality of bacterial isolates wherein at least two of the plurality of bacterial isolates are isolated from faeces of different human donors.
Owner:FINCH THERAPEUTICS HLDG LLC +1
Methods and products for treating gastrointestinal disorders
PendingCN114514027ABacteria material medical ingredientsMicrobiological testing/measurementFecesGastrointestinal disorder
Described herein are compositions and methods for delivering microbial therapeutic agents useful for the treatment of conditions associated with dysbacteriosis of intestinal flora. Described herein are compositions and methods for protecting the gastrointestinal microbiome in a subject. In various embodiments, provided herein are pharmaceutical compositions comprising isolated or purified bacterial isolates and / or mixtures of isolated or purified bacterial isolates (e.g., faeces from humans, e.g., from healthy humans).
Owner:FINCH THERAPEUTICS HLDG LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com