Novel NAMPT enzyme agonist and preparation and application thereof
A technology of activating effect and aromatic compounds, applied in the fields of medicinal chemistry, enzymology and pharmacology, it can solve the problem of not having intellectual property rights, and achieve the effect of good cell protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
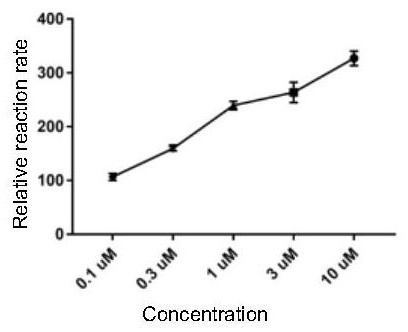
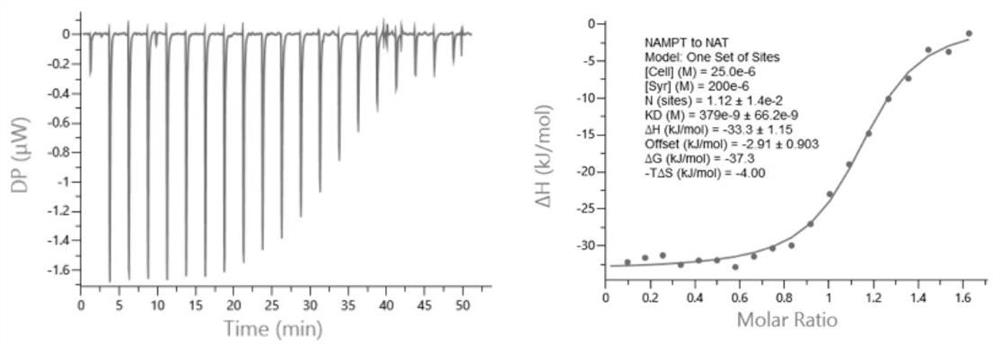
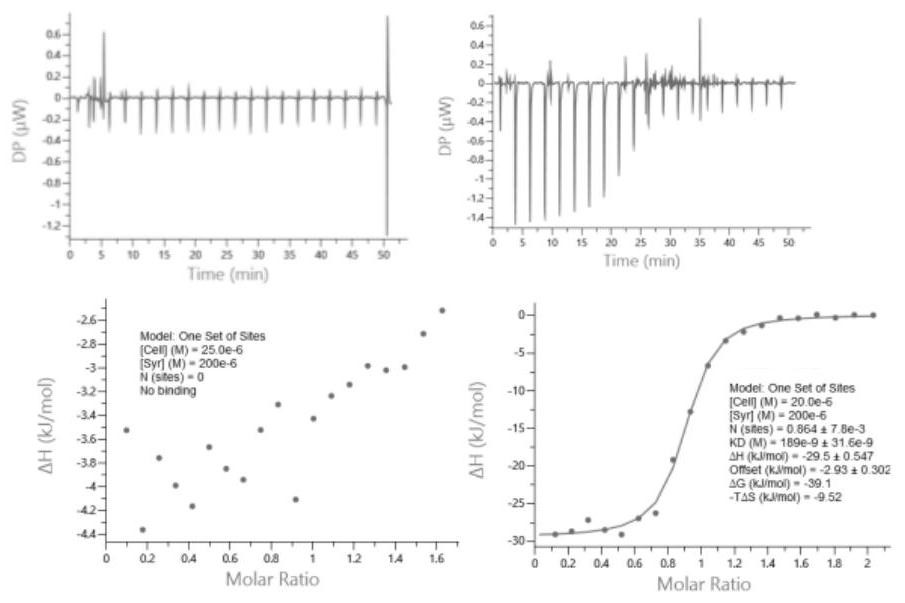
Image
Examples
Embodiment 1
[0068] Example 1. Preparation of 2-(2-(tert-butyl)phenoxy)-N-(4-hydroxyphenyl)acetamide
[0069]
[0070] 1.1 2-(2-(tert-butyl)phenoxy)tert-butyl acetate
[0071] 2-(tert-Butyl)phenol (0.92 g, 6 mmol) and Cs 2 CO 3 (3.9 g, 12 mmol) was dissolved in 6 mL of acetone, then tert-butyl 2-bromoacetate (2.39 g, 12 mmol) was added and the reaction was allowed to overnight at 55°C. After the reaction, the mixture was filtered and the filtrate was concentrated and purified by silica gel chromatography to obtain tert-butyl 2-(2-(tert-butyl)phenoxy)acetate as a white solid. 1 H NMR (400MHz, CDCl 3 )δ7.33(dd,J=7.7,1.7Hz,1H),7.18(ddd,J=8.0,7.3,1.7Hz,1H),6.95(td,J=7.5,1.2Hz,1H),6.74(dd , J=8.1, 1.2Hz, 1H), 4.56(s, 2H), 1.52(s, 9H), 1.45(s, 9H).
[0072] 1.2 2-(2-(tert-butyl)phenoxy)acetic acid
[0073] 2-(2-(tert-Butyl)phenoxy)tert-butyl acetate (0.92 g, 6 mmol) was dissolved in 4 mL of dichloromethane, then 2 mL of trifluoroacetic acid was slowly added dropwise. After stirring the r...
Embodiment 2
[0076] Example 2. Preparation of 2-(2-(tert-butyl)phenoxy)-N-phenylacetamide
[0077]
[0078] Referring to Example 1 (replace 4-aminophenol in step 1.3 with aniline), white solid Example 2 was obtained. 1 HNMR (400MHz, CDCl 3 )δ8.40(s,1H),7.66-7.58(m,2H),7.40(td,J=7.3,1.6Hz,3H),7.28-7.23(m,1H),7.22-7.16(m,1H) ,7.05(td,J=7.6,1.2Hz,1H),6.93(dd,J=8.2,1.2Hz,1H),4.71(s,2H),1.54(s,9H). 13 C NMR (101MHz, CDCl 3 )δ166.43,155.80,138.12,136.99,129.21,127.64,127.30,124.85,122.31,119.69,113.21,68.07,34.77,30.17.
Embodiment 3
[0079] Example 3. Preparation of 2-(2-(tert-butyl)phenoxy)-N-(3-hydroxyphenyl)acetamide
[0080]
[0081] Referring to Example 1 (replace 4-aminophenol in step 1.3 with 3-aminophenol), white solid Example 3 was obtained. 1 H NMR (400MHz, CDCl 3 )δ8.49(s,1H),7.97(t,J=2.2Hz,1H),7.40(dd,J=7.9,1.7Hz,1H),7.35(s,1H),7.24(q,J=8.2 Hz, 2H), 7.05 (td, J=7.6, 1.2Hz, 1H), 6.92 (dd, J=8.2, 1.1Hz, 1H), 6.70 (ddd, J=20.4, 8.0, 2.2Hz, 2H), 4.73 (s,2H),1.52(s,9H). 13 C NMR (101MHz, CDCl 3 )δ167.29,157.32,155.58,138.09,137.72,130.04,127.67,127.35,122.47,113.29,112.34,110.82,107.16,67.82,34.75,30.18.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap