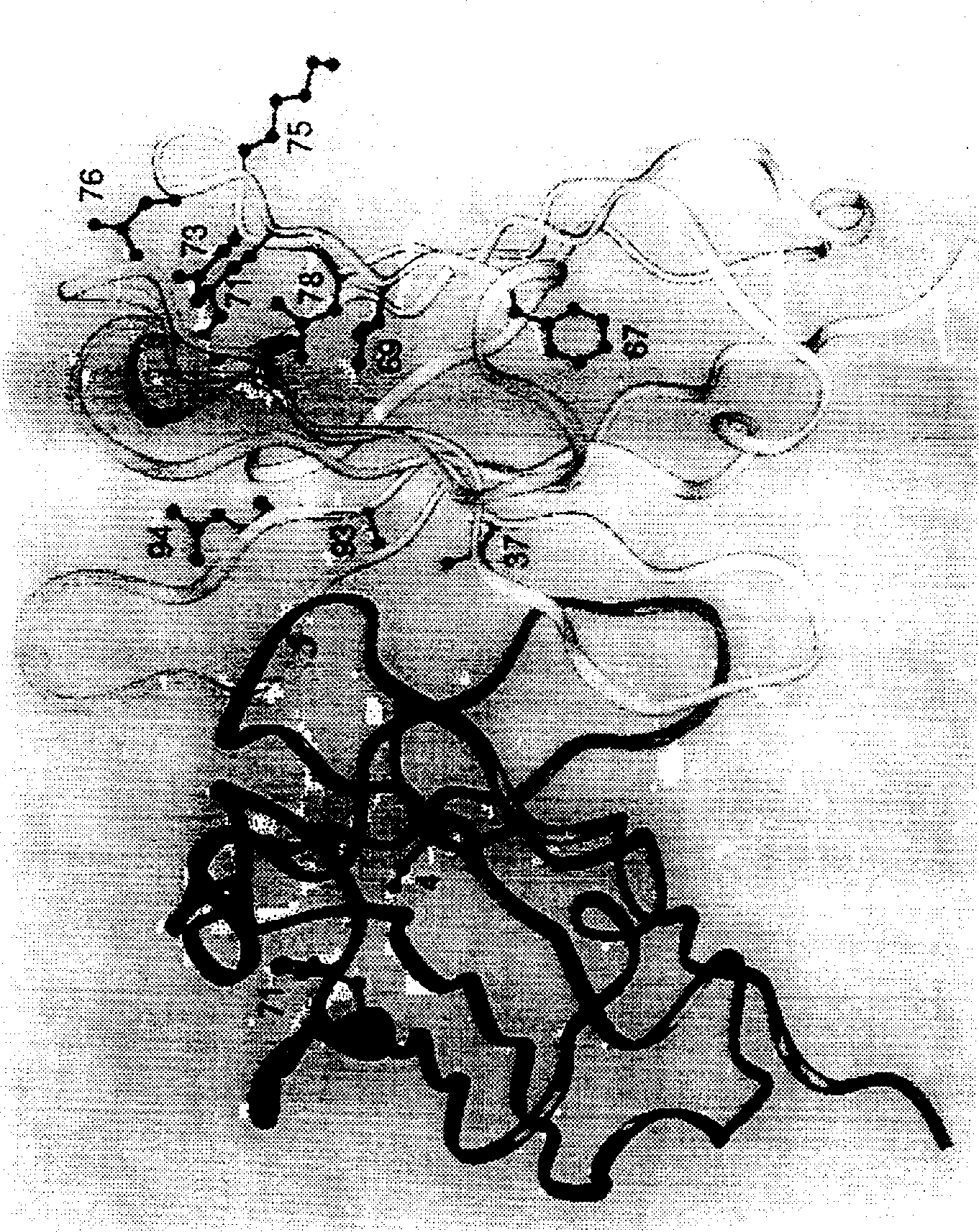Humanized antibodies and method for forming same
A humanized antibody, antibody technology, applied in chemical instruments and methods, biochemical equipment and methods, botanical equipment and methods, etc., can solve problems such as time-consuming, labor-intensive, and unsatisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment I
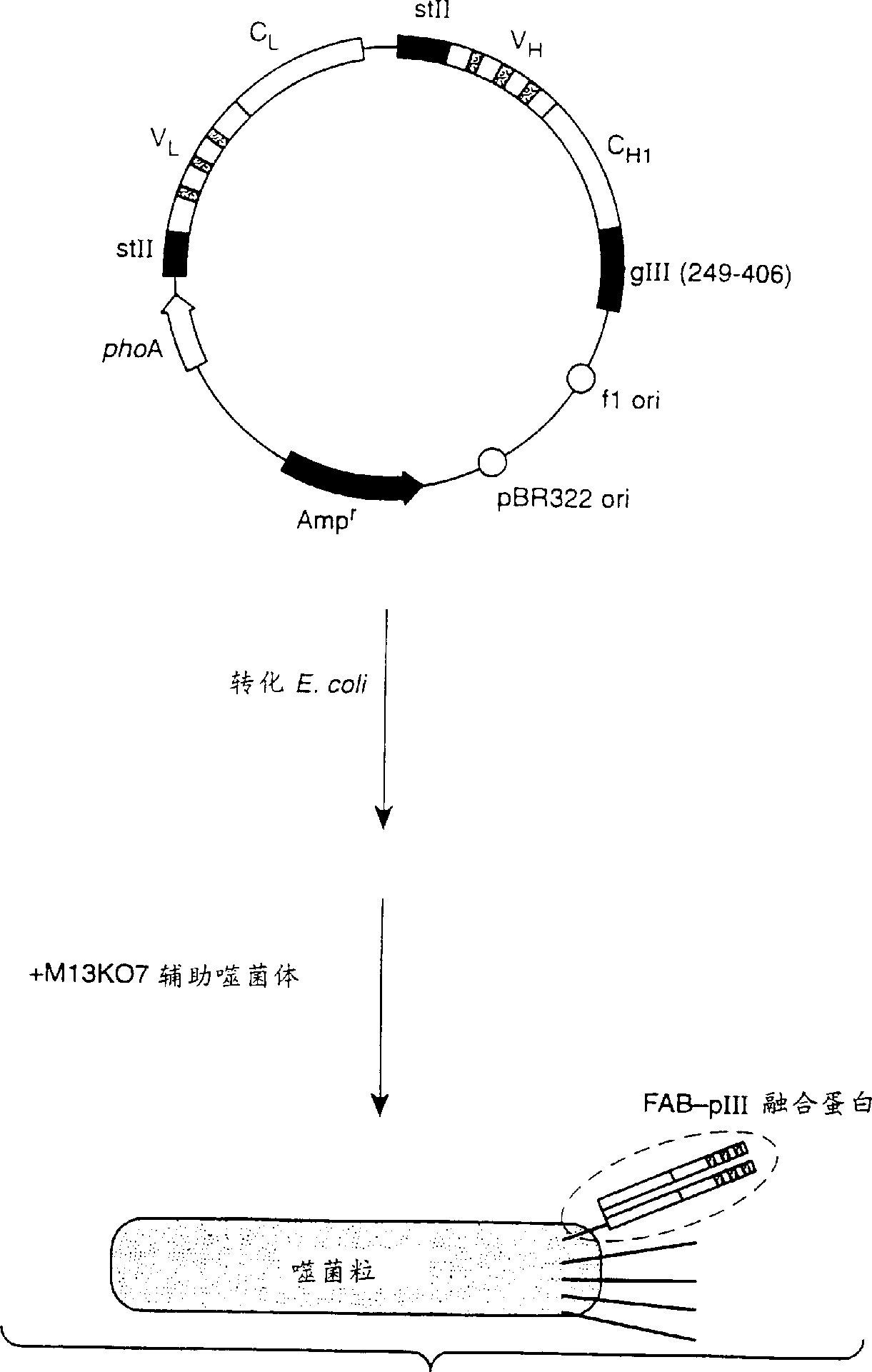
[0067] Construction of phagemid vector and initial humanized anti-VEGF
[0068] The murine anti-VEGF monoclonal antibody A4.6.1 has already been described by Kim et al., Growth Factors 7, 53 (1992); Kim et al., Nature 362, 841 (1993). The first humanized Fab variant hu2.0 of A4.6.1 was constructed as follows: L κI-Cκ 1 light chain and human V H III-C H lγ 1 The deoxyuridine-containing template of the plasmid pAK2 for the Fd fragment of the heavy chain was subjected to site-directed mutagenesis (Carter et al., Proc. Natl. Acad. Sci. USA 89, 4285 (1992)). According to the sequence definitions in Kabat et al., Sequences of Proteins of Immunological Interest, (5th), Public Health Service, National Institutes of Health, Bethesda, MD (1991), the CDR sequences of A4.6.1 to be transplanted were selected, except CDR- In addition to H1, we extended CDR-H1 to include sequence and structural sequence, ie V H Residues 26-35 (Chothia et al., J. Mol. Biol. 196, 901 (1987)). The Fab co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com