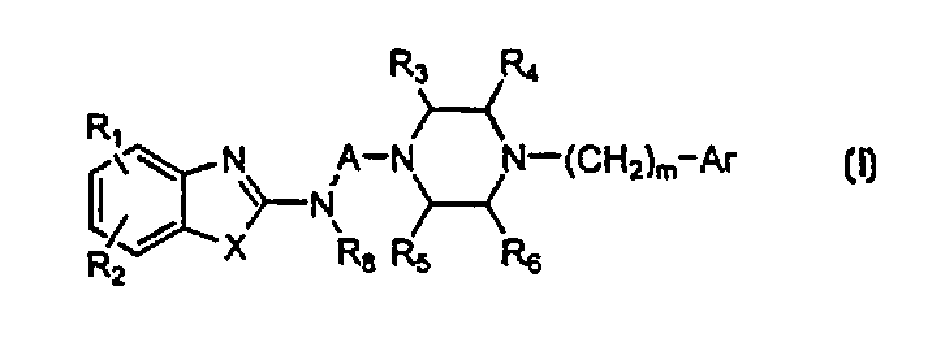
2-piperazino alkylatnino benzoazole derivatives: dopamine receptor subtype sepcific ligands
A technology of alkylamino and alkylsulfonyl, which is applied in the field of pharmaceutical compositions containing the compound, and can solve problems such as delayed dyskinesia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] 1-(5-fluoropyrimidin-2-yl)-4-(4-aminobutyl)piperazine
[0110] At 80°C, 4-bromo-N-butylphthalimide (8.37g) and 1-(5-fluoropyrimidin-2-yl)piperazine (5.4g) containing potassium carbonate (8.2g) A solution of dimethylformamide (100 mL) was stirred for 12 hours. After cooling, the mixture was poured into water and extracted with ether. The ether layer was dried over sodium sulfate, filtered and concentrated to give the intermediate as a yellow solid. The resulting phthalimide was then dissolved in hydrazine monohydrate (100ml) and refluxed under nitrogen overnight. After cooling, the mixture was poured into a 30% solution of potassium carbonate (500ml) and extracted with diazomethane, dried and concentrated to give an orange semi-solid (4.66g). This material was dissolved in a 10% methanol / isopropanol mixture (50ml), treated with fumaric acid (4.27g, 2eq) and the solvent volume reduced to 20ml. The resulting yellow crystals (6.5 g) were collected by filtration.
Embodiment 2
[0112] 1-(5-fluoropyrimidin-2-yl)-4-(2-[6-benzothiazol-2-ylamino]butyl)piperazine hydrogen fumarate
[0113]Acetonitrile solution (10 mL) of 2-chlorobenzothiazole (920 mg) and 1-(5-fluoropyrimidin-2-yl)-4-(4-aminobutyl)piperazine (254 mg) ) was refluxed under nitrogen for 10 hours. After cooling, the mixture was concentrated and the resulting residue was partitioned between ethyl acetate and water. The organic phase was separated and extracted with 10% citric acid. The acidic aqueous phase was basified with 10N NaOH solution and extracted with chloroform. The chloroform layer was then dried over sodium sulfate, filtered and concentrated to give the title compound as a white solid (0.31 g). [also known as benzothiazol-2-yl{4-[4-(5-fluoropyrimidin-2-yl)piperazinyl]butyl}amine]. This material was dissolved in 10% methanol / isopropanol and treated with fumaric acid (190 mg). The solvent volume was reduced a portion and the resulting crystals (347 mg, m.p. 168-170°C) were iso...
Embodiment 3
[0115] 1-(2-Methylhydrophenyl)-4-(2-[6-fluorobenzothiazol-2-ylamino]ethyl)piperazine hydrogen fumarate
[0116] A solution of 6-fluoro-2-aminobenzothiazole (5 g) and triethylamine (5 ml) in chloroform (100 ml) was stirred vigorously during the dropwise addition of a solution of chloroacetyl chloride (5 ml) in chloroform (10 ml). The reaction mixture was stirred overnight, filtered and concentrated. The residue was triturated with isopropanol to give a white solid (3.82g).
[0117] A portion of this solid (150mg, 0.61mmol) was dissolved in acetonitrile (10ml) and phased. To the resulting solution were added 1-(2-methoxyphenyl)piperazine (118mg) and potassium carbonate (150mg). The mixture was refluxed overnight. After cooling, the solvent was removed and the resulting residue was partitioned between ethyl acetate and water. The organic phase was dried and evaporated to give a yellow oil which was purified by preparative thin layer chromatography eluting with 9% methanol / ch...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap