Peptide-deformylase inhibitors
A formyl hydroxylamine group, methyl group technology, applied in the field of peptide deformylase inhibitors, can solve the problems of high sequence polymorphism and low consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 2 approach
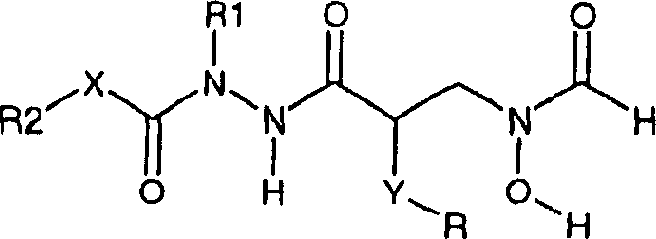
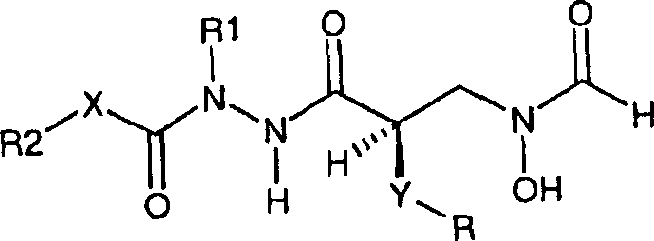
[0088] As the second embodiment of the present invention, the compound of X=O in the formula (1) is disclosed, namely racemic compound (34) and chiral compounds (36) and (38). These compounds preferably have R1=H.
[0089]
[0090] Preferred compounds for use in the present invention are selected from the following group of compounds:
[0091] N-Butyl-N-(tert-butoxycarbonyl)-N'-{(2R)-[(formylhydroxylamino)methyl]-heptanoyl}hydrazine;
[0092] N-butyl-N-phenoxycarbonyl-N'-{(2R)-[(formylhydroxylamino)methyl]-heptanoyl}-hydrazine;
[0093] N-isobutyl-N-(tert-butoxycarbonyl)-N'-{(2R)-[(formylhydroxylamino)methyl]heptanoyl}-hydrazine;
[0094] N-isobutyl-N-phenoxycarbonyl-N'-{(2R)-[(formylhydroxylamino)methyl]heptanoyl}-hydrazine;
[0095] N-phenethyl-N-(tert-butoxycarbonyl)-N'-{2-[(formylhydroxylamino)methyl]heptanoyl}-hydrazine;
[0096] N-cyclohexylmethyl-N-(tert-butoxycarbonyl)-N'-{2-[(formylhydroxylamino)methyl]heptanoyl}-hydrazine;
[0097] N-Benzyl-N-(tert-butoxycarb...
Embodiment 1
[0210] N-Butyl-N-(tert-butoxycarbonyl)-N'-{(2R)-[(formylhydroxylamino)methyl]heptanoyl}-hydrazine
[0211] 10% Pd / C (60 mg) was added to tert-butyl N'-{(R)-2-[(benzyloxycarboxamido)methyl]heptanoyl}-N-butylhydrazinecarboxylate (195 mg, 0.421 mmol) in ethanol (15 ml) solution. The reaction solution was hydrogenated overnight at room temperature. After the reaction was completed, the reaction solution was filtered through a pad of Celite and washed with ethanol (10 mL×2). The solvent was removed to obtain the crude product, and the crude product was purified by high performance liquid phase to obtain 52 mg (33%) of the target compound
[0212] 1 HNMR (400MHz, CDCl 3 ): δ9.94(s, 1H), 9.39(s, 1H), 8.32(s, 1H), 4.10(dd, J=14.1, 4.0Hz, 1H), 3.62(m, 1H), 3.35(m, 2H), 2.55(m, 1H), 1.72(m, 1H), 1.56(m, 1H), 1.50(s, 9H), 1.30(m, 10H), 0.90(m, 6H).
[0213] MH+374.
[0214] Preparation 12
[0215] Phenyl N-butylhydrazine carboxylate
[0216]Phenyl chloroformate (0.53 mL, 4.20 mm...
Embodiment 2
[0220] N-Butyl-N-phenoxycarbonyl-N'-{(2R)-[(formylhydroxylamine and)methyl]heptanoyl}-hydrazine
[0221] Purification by preparative HPLC yielded 49 mg (41%) of the title product.
[0222] 1 HNMR (400MHz, CDCl 3 ): δ9.78(s, 1H), 9.39(s, 1H), 8.27(s, 1H), 7.40-7.10(m, 5H), 4.20-3.30(m, 4H), 2.70(m, 1H), 1.80-1.20(m, 10H), 0.90(m, 6H).
[0223] MH+394.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com