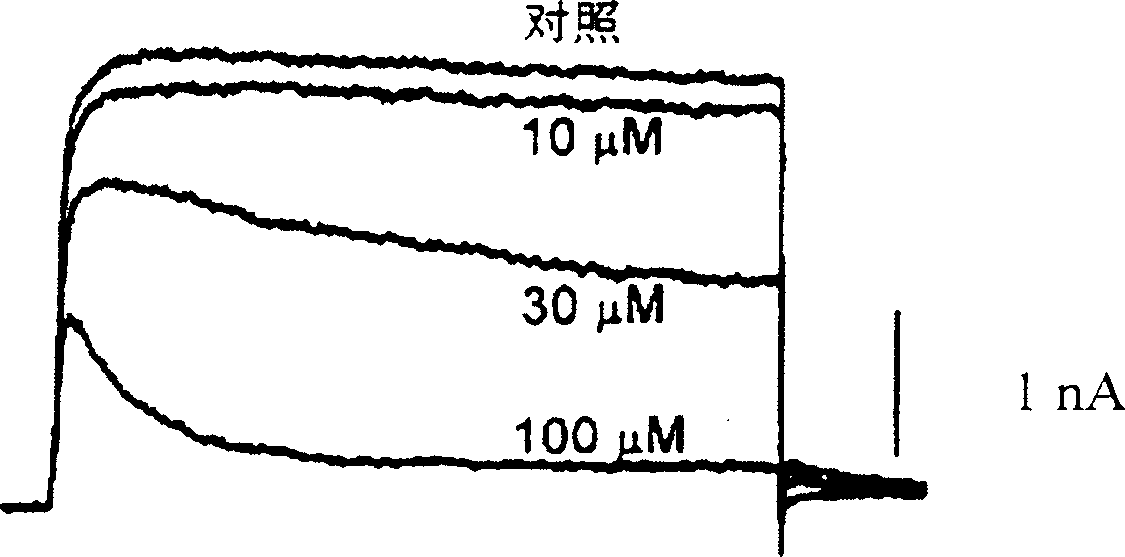
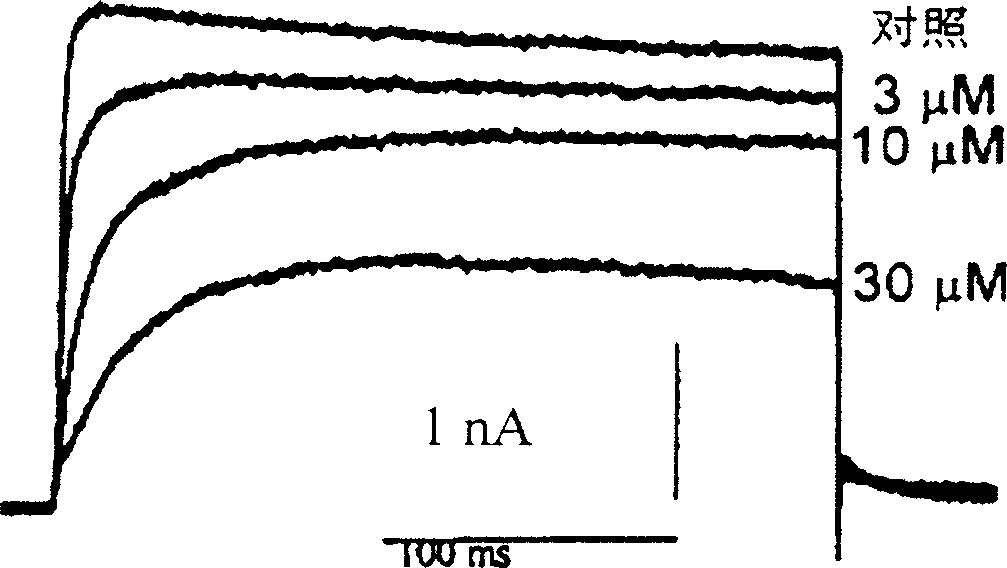
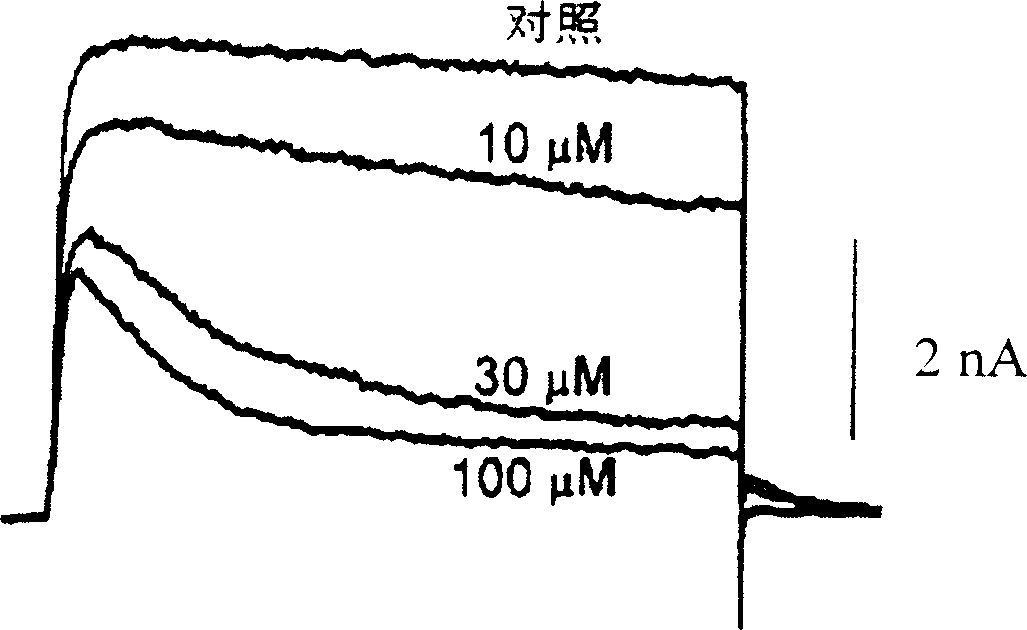
Pharmaceutical compositions comprising chelidonine or derivatives thereof
A technology of chelidonine and composition, applied in the field of pharmaceutical compositions containing chelidonine or its derivatives, capable of solving the problems of lack of ion channel selectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Example 1: Preparation of (12bR)-13,14-dihydro-13-methyl[1,3]benzodioxolo[5,6c]-1,3-dioxolo[4,5- i]phenanthridine (compound 6)
[0111] Dess-Martin Periodinane (25mg, 0.059mmol) was dissolved in anhydrous dichloromethane (0.2ml), then stirred at room temperature for 10 minutes. A solution of chelidonine (20 mg, 0.054 mmol) in anhydrous dichloromethane (0.1 ml) was added dropwise to the reaction solution. After stirring for 30 minutes, diethyl ether (0.9 ml) was added to the reaction solution. The reaction solution was added to a mixture of saturated potassium carbonate aqueous solution (0.56 ml) and sodium thiosulfate pentahydrate (0.1 g), followed by stirring for 15 minutes. The reaction solution was extracted with ethyl acetate, washed, dried and purified by column chromatography (hexane:ethyl acetate=10:1) to prepare 6.6 mg (37%) of the title compound (Compound 6). At this point, 9 mg (45%) of chelidonine were recovered.
[0112] R f = 0.24 (hexane: ethyl acetat...
Embodiment 2
[0114]Example 2: Preparation of (12bR)-7,12b,13,14-tetrahydro-13-methyl[1,3]-benzodioxolo[5,6c]-1,3-dioxolo [4,5-i]phenanthridine (Compound 7)
[0115] A solution of compound 10 (20 mg, 0.046 mmol) in dry methanol (0.3 ml) was stirred at room temperature for 10 minutes, and then potassium tert-butoxide (15 mg, 0.138 mmol) was added thereto. The reaction solution was stirred at room temperature for 13 hours. The reaction solution was extracted with ethyl acetate, washed, dried, and purified by column chromatography (hexane:ethyl acetate=4:1) to prepare 10 mg (64%) of the title compound (Compound 7).
[0116] R f =0.22 (hexane:ethyl acetate=4:1)
[0117] 1 H-NMR (500MHz, CDCl 3 ): δ7.16(d, 1H, J=6.0Hz), 7.16(d, 1H, J=8.0Hz), 6.74(d, 1H, J=8.0Hz), 6.60(s, 1H), 6.50-6.48 (m, 1H), 5.97(d, 2H, J=10Hz), 5.93(d, 2H, J=10Hz), 4.56-4.53(m, 1H), 4.43(d, 2H, J=16.5Hz), 3.92 (d, 2H, J=16.5Hz), 3.59-3.41 (m, 2H), 1.99 (s, 3H).
Embodiment 3
[0118] Example 3: Preparation of [5bR-(5bα, 6β, 12bα)]-5b, 6, 7, 12b, 13, 14-hexahydro-13-methyl[1,3]benzodioxolo[5, 6c]-1,3-dioxolo[4,5-i]phenanthridine (Compound 8)
[0119] 10% palladium (1 mg) was added to compound 7 (10 mg, 0.030 mmol) in dry methanol (0.3 ml) and stirred at room temperature for 10 minutes. Hydrogen was added while the reaction solution was stirred for 1 hour. The reaction solution was filtered through a celite filter pad, concentrated under reduced pressure, and purified by column chromatography (hexane:ethyl acetate=6:1) to prepare 2 mg (20%) of the title compound 8 and 1.8 mg (18%) of compound 6. mixture.
[0120] R f = 0.27 (hexane: ethyl acetate = 6: 1)
[0121] 1 H-NMR (500MHz, CDCl 3 ): δ6.93(s, 1H), 6.75(d, 1H, J=8.5Hz), 6.72(d, 1H, J=8.5Hz), 6.56(s, 1H), 5.94(d, 2H, J=8.5Hz) 1 5Hz), 5.92(d, 2H, J=3Hz), 3.94(d, 1H, J=16.5Hz), 3.61(d, 1H, J=4.0Hz), 3.48(d, 1H, J=16Hz), 3.07-3.04 (m, 1H), 2.79-2.67 (m, 2H), 2.40 (s, 3H), 1.91-1.88 (m, 1H). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com