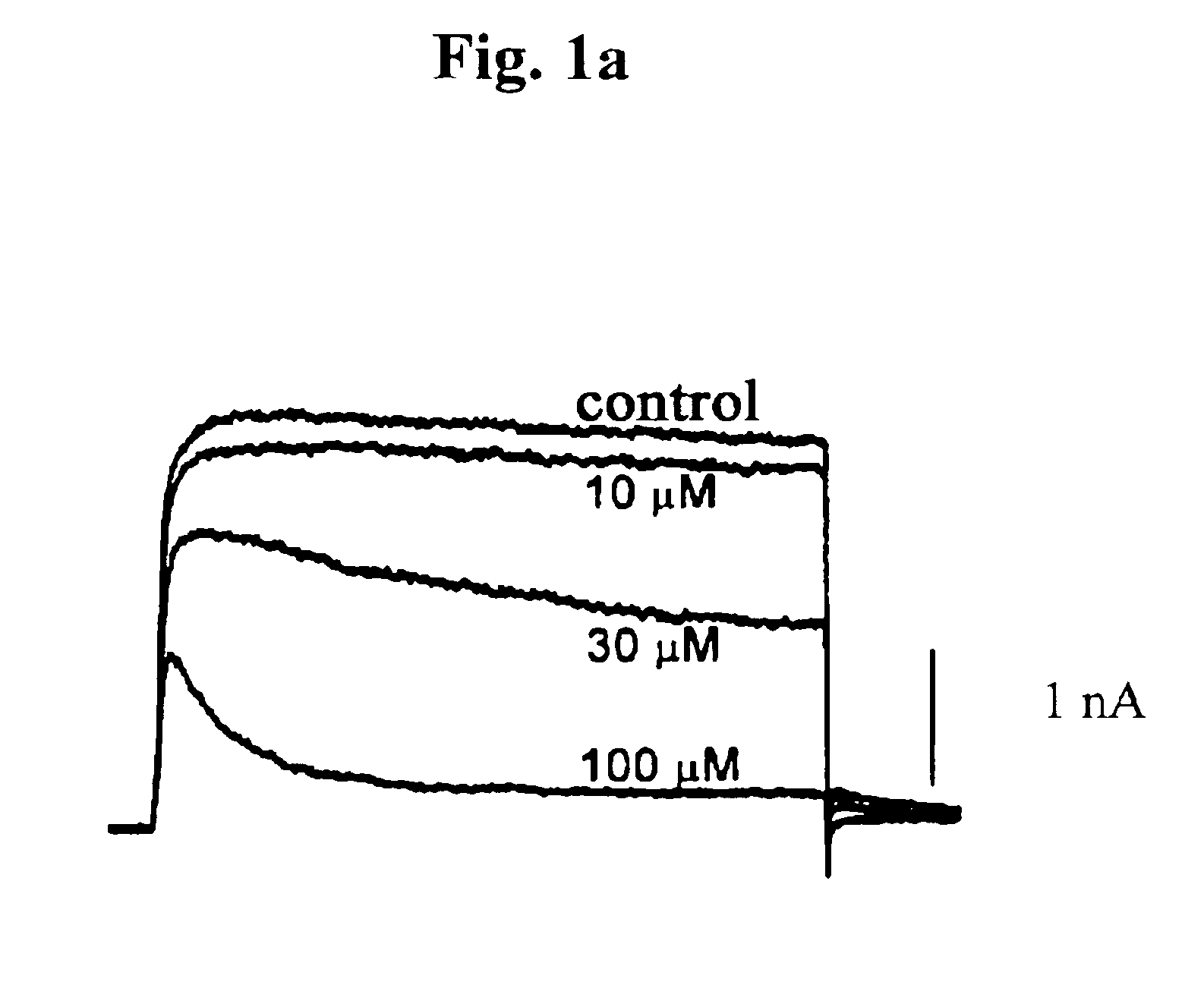
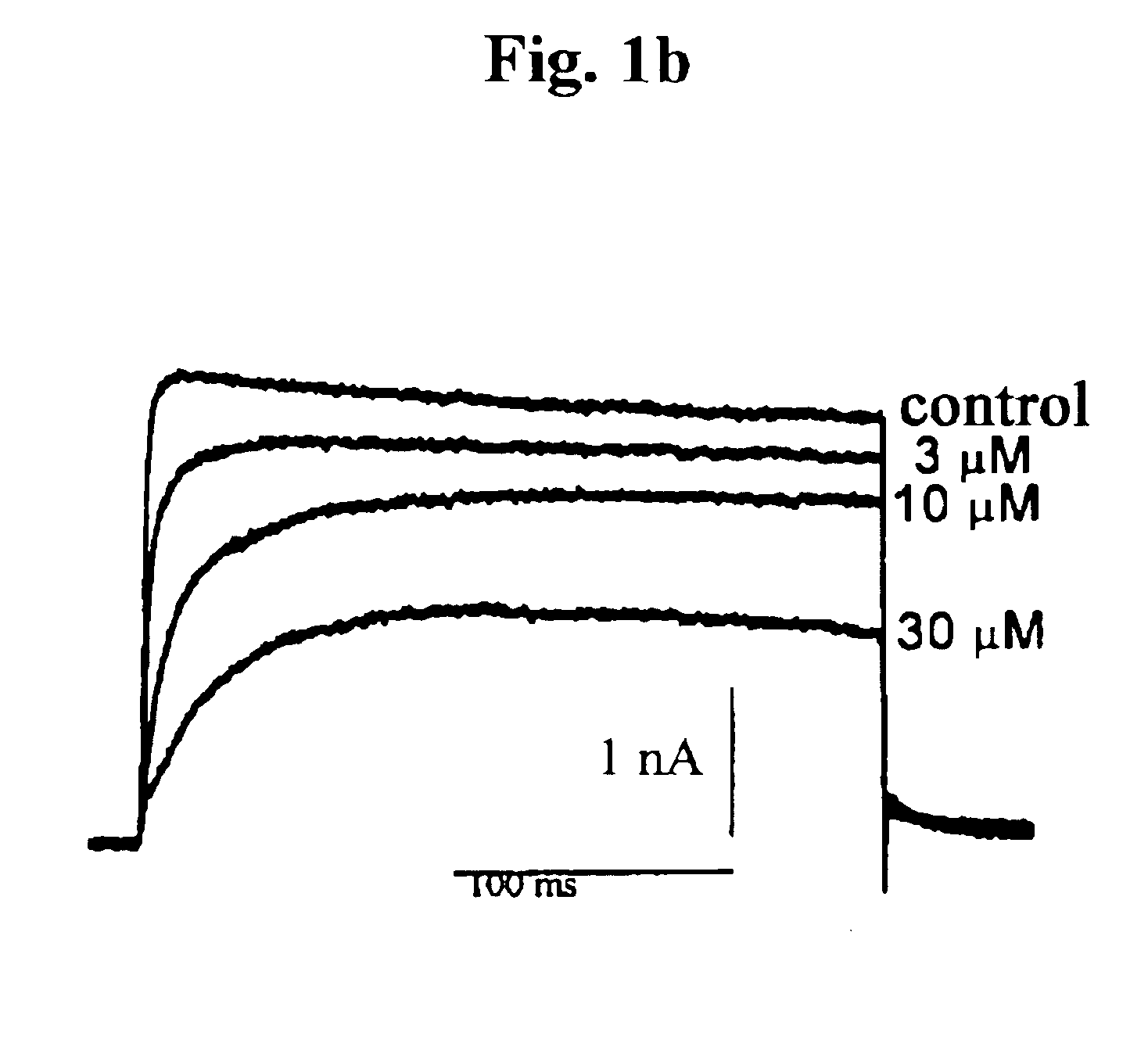
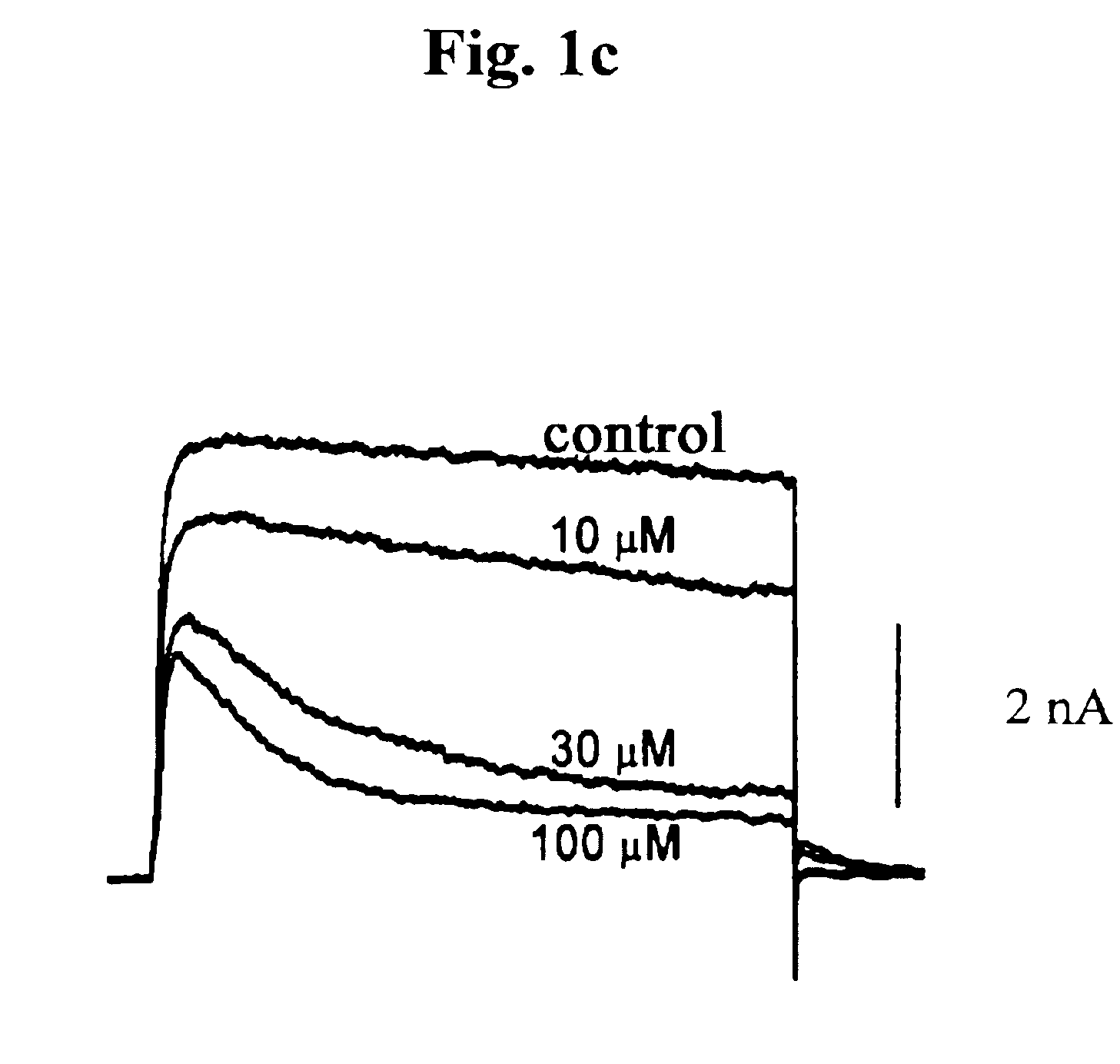
Pharmaceutical compositions comprising chelidonine or derivatives thereof
a technology of chelidonine and composition, applied in the direction of drug composition, biocide, cardiovascular disorder, etc., can solve the problems of abnormal cardiac impulse formation or impulse propagation, less effective heart pump, and use of drugs to manage cardiac arrhythmias
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 5
Preparation of {[5bR-(5bα,6β,12bα)]-5b,6,7,12b,13,14-hexahydro-13-methyl[1,3]benzodioxolo[5,6c]-1,3-dioxolo[4,5-i]phenanthridin-6-yl}-methanesulfonate (compound 10)
[0108]Chelidonine (18 mg, 0.048 mmol) was dissolved in anhydrous methylene dichloride (0.5 ml), and then stirred at 0° C. for 10 minutes. Anhydrous triethylamine (8 μl, 0.058 mmol) and methanesulfonyl chloride (6 μl, 0.073 mmol) were added to the solution, and stirred at room temperature for 6 hours. The resulting reaction solution was extracted with ethylacetate, washed, dried, and purified by column chromatography (hexane:ethylacetate=2:1) to prepare 16 mg (77%) of the title compound (compound 10).
[0109]Rf=0.39 (hexane:ethylacetate=2:1)
[0110]1H-NMR (500 MHz, CDCl3): δ 7.21 (d, 1H, J=8.0 Hz), 7.18 (s, 1H), 6.69 (d, 1H, J=8.5 Hz), 6.42 (s, 1H), 5.90 (d, 2H, J=5.0 Hz), 5.88 (d, 2H, J=15.5 Hz), 5.18-5.16 (m, 1H), 4.13-4.11 (m, 1H), 3.75 (s, 1H), 3.73 (d, 2H, J=12 Hz), 3.45 (d, 2H, J=17 Hz), 3.14-3.08 (m, 1H), 3.05 (s, 3H), ...
example 6
Preparation of {[5bR-(5bα,6β,12bα)]-5b,6,7,12b,13,14-hexahydro-13-methyl[1,3]benzodioxolo[5,6c]-1,3-dioxolo[4,5-i]phenanthridin-6-yl}-4-methylbenzenesulfonate (Compound 11)
[0111]A solution of chelidonine (10 mg, 0.027 mmol) in anhydrous methylene dichloride (0.3 ml) was stirred at 0° C. for 10 minutes. Anhydrous triethylamine (5 μl, 0.032 mmol) and paratoluenesulfonyl chloride (8 μl, 0.040 mmol) were added to the solution, and stirred at room temperature for 12 hours. The resulting reaction solution was extracted with ethylacetate, washed, dried, and purified by column chromatography (hexane:ethylacetate=2:1) to prepared 8.6 mg (63%) of the title compound (compound 11).
[0112]Rf=0.36 (hexane:ethylacetate=2:1)
[0113]1H-NMR (500 MHz, CDCl3): δ 7.86 (d, 2H, J=8.5 Hz), 7.37 (d, 2H, J=8.5 Hz), 7.30-7.26 (m, 1H), 7.20 (s, 1H), 6.66 (d, 2H, J=8.0 Hz), 6.33 (s, 1H), 5.89 (d, 2H, J=3.5 Hz), 5.87 (d, 2H, J=18 Hz), 4.96-4.92 (m, 1H), 4.03 (d, 1H, J=5.0 Hz), 3.67 (d, 2H, J=17.5 Hz), 3.60 (bs, 1H)...
example 7
Preparation of {[5bR-(5bα,6β,12bα)]-5b,6,7,12b,13,14-hexahydro-13-[1,3]benzodioxolo[5,6c]-1,3-dioxolo[4,5-i]phenanthridin-6-yl}-carbamate (Compound 12)
[0114]A solution of sodium carbonate (10 mg, 0.097 mmol) and chlorosulfonyl isocyanate (6 μl, 0.065 mmol) in anhydrous methylene dichloride (0.4 ml) was stirred at −78° C. for 10 minutes. A solution of chelidonine (20 mg, 0.054 mmol) in anhydrous methylene dichloride (0.14 ml) was added dropwise to the reaction solution. After the resulting reaction solution was stirred for 1 hour and slowly heated to 0° C., the reaction was quenched with water. The reaction solution was brought to pH >7 with 3N aqueous sodium hydroxide solution. Subsequently, the reaction solution was extracted with ethylacetate, washed, dried and purified by column chromatography (hexane:ethylacetate=1:1) to prepare 5.4 mg (20%) of the title compound (compound 12). At this time, 4 mg (40%) of chelidonine was recovered.
[0115]Rf=0.31 (hexane:ethylacetate=1:1)
[0116]1H-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com