Method for controlling quality of Chinese medicinal composition to treat cancerous pain and application thereof
A quality control method and composition technology, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as stagnation and unappeared varieties of traditional Chinese medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1: the preparation of composition injection
[0086] Take Qingfengteng 800g, celandine 700g and Chinese peach leaves 1000g three flavors, celandine and Qingfengteng add 8 times the amount of 80% ethanol to soak for 30 minutes, heat and reflux twice, each time for 2 hours, combine the alcohol extracts, Recover ethanol under reduced pressure and concentrate to 1:1, adjust pH 2-3 with 1mol / L phosphoric acid, let it stand for 12 hours, filter, adjust pH 10-11 with 40% sodium hydroxide solution, and extract 5 times with equal amount of chloroform, The chloroform solutions were combined, the chloroform was recovered, and the residue was vacuum-dried to obtain the total alkaloids. Add water to decoct Han peach leaves twice, each time for 1.5 hours, combine the decoction liquid, filter, concentrate the filtrate under reduced pressure, and use ethanol precipitation for two times. The alcohol content of the first solution reaches 60%, and the second time reaches 85%. ...
Embodiment 2
[0087] Embodiment 2: the identification of injection
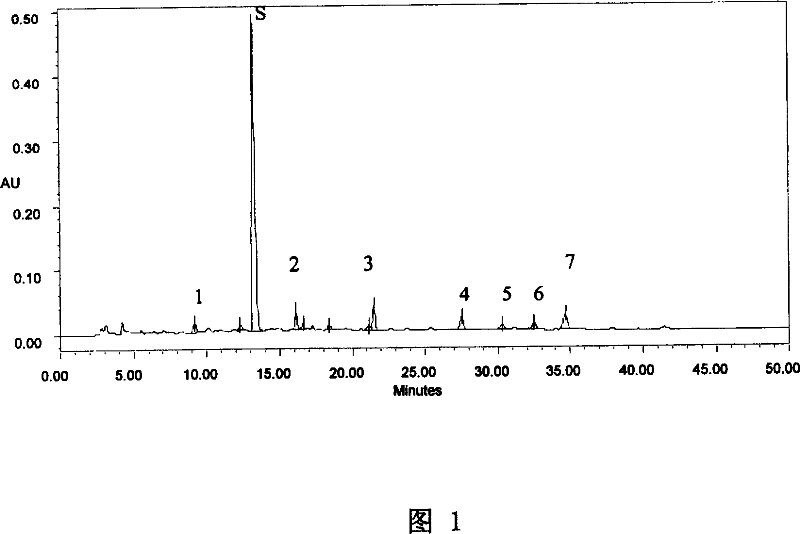
[0088] A. Take 10ml of this pharmaceutical composition injection, adjust the pH value to 9-10 with 10% sodium hydroxide solution, add chloroform to extract 2 times, 10ml each time, combine the chloroform solution, evaporate to dryness, add 2ml of methanol to the residue to dissolve, as The test solution; take another 2g of Qingfengteng reference medicinal material and 5g of celandine reference medicinal material, add 1% hydrochloric acid solution 30ml and 50ml respectively, ultrasonically treat for 40 minutes, filter, add 10% sodium hydroxide solution to the filtrate to adjust the pH value To 9~10, add chloroform to prepare the reference medicinal material solution in the same way; then take sinomenine and chelidonine reference substances, add methanol respectively to make solutions containing 2mg and 1mg per 1ml, as the reference substance solution; according to thin-layer chromatography method test, draw 6 μl of each o...
Embodiment 3
[0090] Embodiment 3: the content determination of injection
[0091] A. Determination of sinomenine content
[0092] Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as filler, use 15:85 acetonitrile-0.02M potassium dihydrogen phosphate as mobile phase: detection wavelength 265nm Theoretical plate number is based on sinomenine peak The calculation should not be less than 2000;
[0093] Preparation of reference substance solution: Accurately weigh an appropriate amount of sinomenine reference substance, add methanol to make a solution containing 0.5mg per 1ml, shake well, and obtain;
[0094] Preparation of the test solution: Accurately measure 1ml of the pharmaceutical composition injection, put it in a 10ml measuring bottle, add methanol to dilute to the mark, shake well, filter through a 0.45 μm microporous membrane, and use the filtrate as the test solution;
[0095] Determination method: Precisely draw 10 μl each of the refe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com