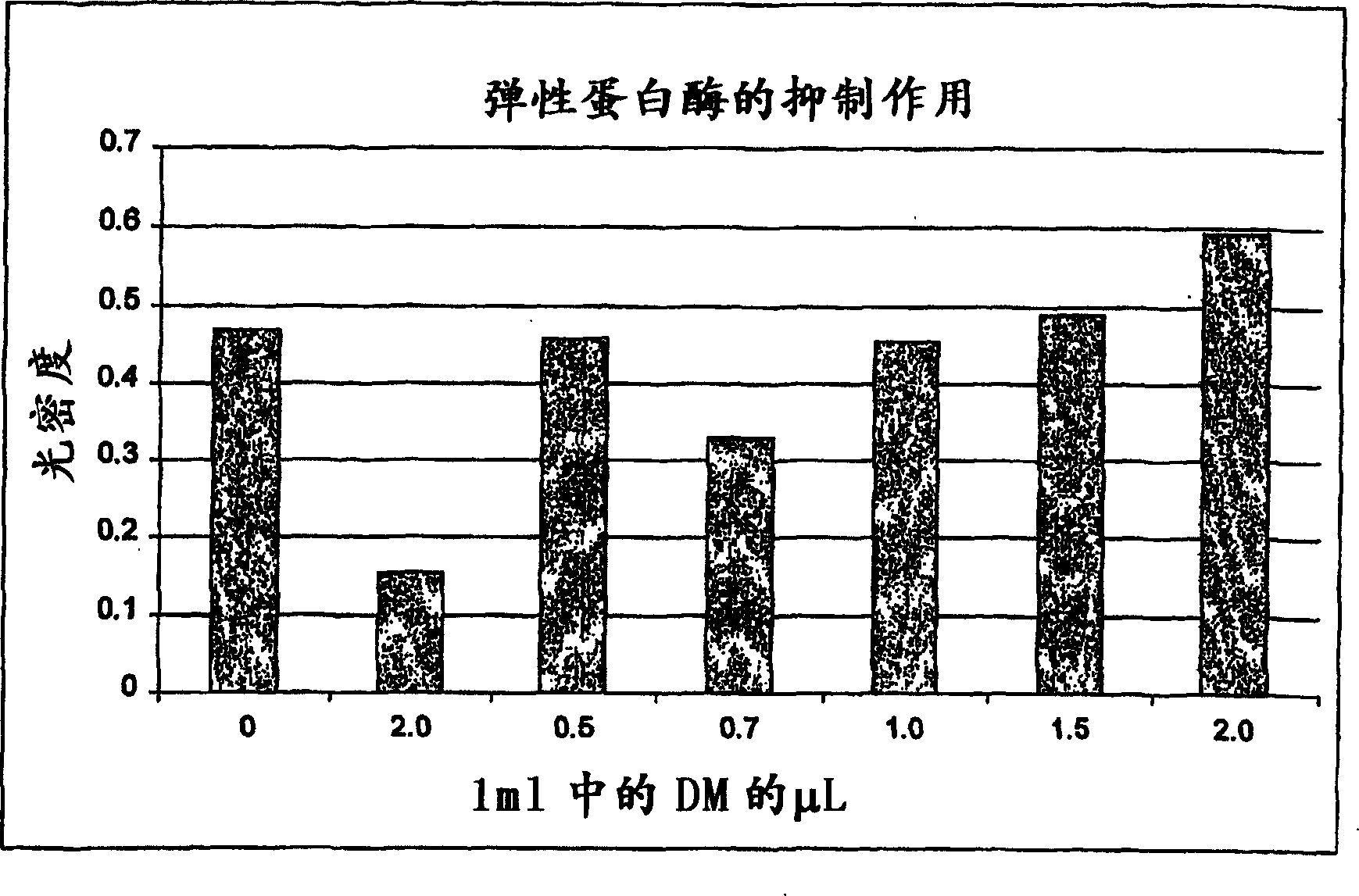
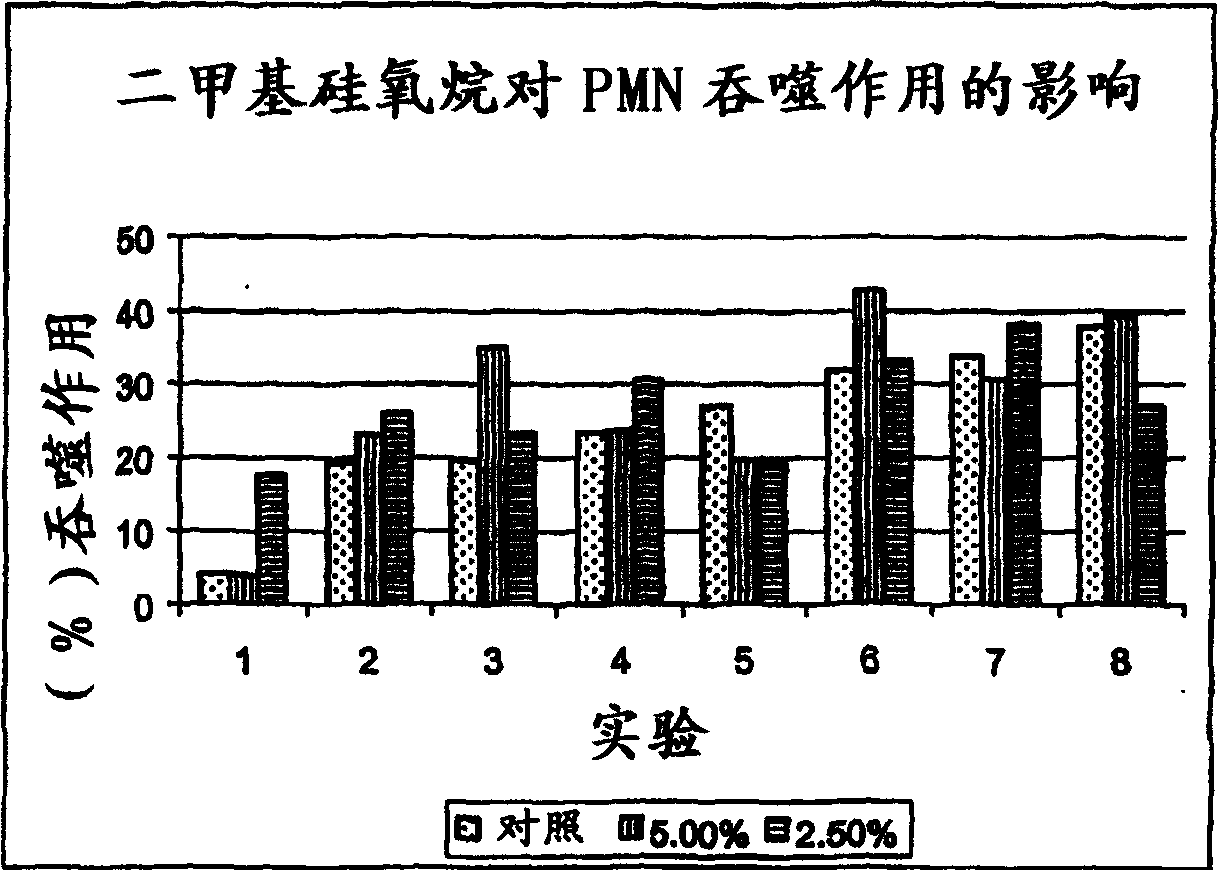
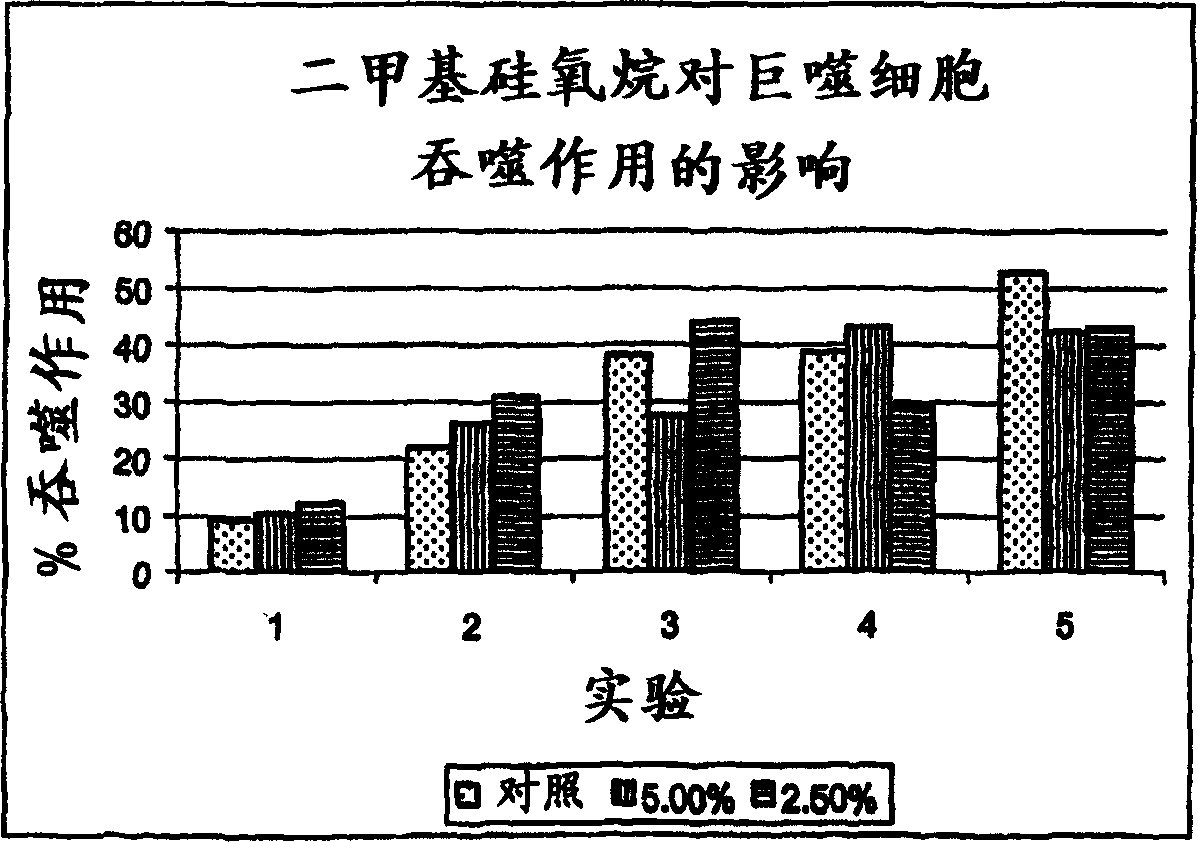
Method for the treatment of inflammation
A technology of inflammation and anti-inflammatory drugs, applied in the fields of immunology and pharmacology, can solve problems such as hypertension and nephrotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of Dimethicone Oil
[0039] An oil composition containing dimethylsiloxane was prepared using the formulation described in Table 1 as follows.
[0040] Table 1
[0041] Dimethicone Composition Ingredients
[0042] Composition % weight
[0043] Dimethicone 10.0
[0044] Heavy mineral oil (Ramol 350) 79.4
[0045] Isopropyl myristate (emulsifier) 10.0
[0046] Lanolin alcohol (Amerchol L-101) 0.5
[0047] Sorbic acid 0.1
[0048] Total 100.0
[0049] Isopropyl myristate (INOLEX CHEMICAL CO) was heated to 54-60°C in a stainless steel vessel. Add sorbic acid (PENTA MANUFACTURING COMPANY) and mix until completely dissolved. Add AMERCHOL L-101 (CHARLES TENNANT COMPANY CANADAL TD) and mix, adjust temperature to 50-55°C and continue mixing until formulation is uniform. Dimethicone (SILICONE SF 96-350, GENERAL ELECTRIC COMPANY) and heavy mineral oil (CANADA COLORS AND CHEMICALS) were added ...
Embodiment 2
[0051] Preparation of Dimethicone Cream
[0052] A cream composition containing dimethicone was prepared using the formulation described in Table 2 as follows.
[0053] Table 2
[0054] Dimethicone Composition Ingredients
[0055] Composition % weight
[0056] Dimethicone 5.00
[0057] Glycerin 8.00
[0058] Anhydrous citric acid 0.06
[0059] Edetate Disodium 0.05
[0060] Atmul 84 4.00
[0061] Lanolin oil (Amerchol L-101) 3.00
[0062] Cholesterol 0.10
[0063] Emulgade 1000 8.00
[0064] Eumulgin B1 0.50
[0065] Purified water 71.29
[0066] Total 100.00
[0067] Oil Phase (Phase A):
[0068] Mix Atmul 84 (VAN WATERS & ROGERS), Amerchol L-101, Cholesterol (CRODA CANADA LTD, Canada), Emulgade 1000 (HENKEL), Eumulgin B1 (HENKEL), Dimethicone in a heating pot at 72-78°C alkanes (Silicone SF96-350, GENERAL ELECTRIC COMPANY, Canada).
[0069] Aqueous Phase (Phase B):
[0070] Purified water and g...
Embodiment 3
[0072] Preparation of Dimethicone Cream Containing Caprylic / Capric Triglycerides
[0073] A dimethicone cream composition containing caprylic / capric triglyceride was prepared as follows using the formulation described in Table 3 below.
[0074] table 3
[0075] Dimethicone Composition Ingredients
[0076] Composition % weight
[0077] Dimethicone 5.00
[0078] Caprylic / Capric Triglyceride 5.00
[0079] Glycerin 8.00
[0080] Citric acid (anhydrous) 0.06
[0081] Edetate disodium 0.05
[0082] Atmul 84 4.00
[0083] Lanolin oil (Amerchol L-101) 3.00
[0084] Cholesterol 0.10
[0085] Emulgade 1000 8.00
[0086] Eumulgin B1 0.50
[0087] Purified water 66.29
[0088] Total 100.00
[0089] Oil Phase (Phase A):
[0090] Atmul 84, Amerchol L-101, Cholesterol, Emulgade 1000, Eumulgin Bl, Caprylic / Capric Triglyceride (Croda Canada Ltd, Canada) and Dimethicone were mixed in a heating jug at 72-78°C.
[0091] Aqueous Phase (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com