Chalcone derivatives and their use to treat diseases
A technology of compound, alkyl, applied in the field of new chalcone derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 6c
[0603] Embodiment 6c. Amide Moiety
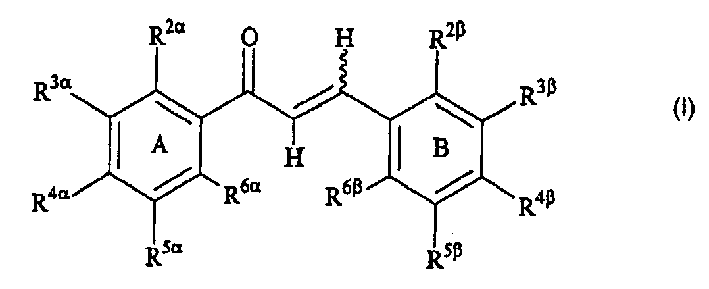
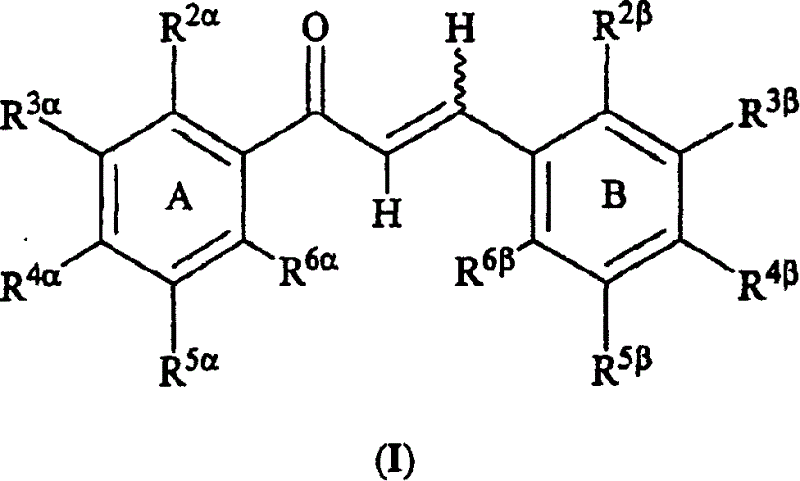
[0604] In a 61st embodiment, the invention is represented by Formula I or a pharmaceutically acceptable salt or ester thereof, wherein:
[0605] R 2β , R 3β , R 4β , R 5β , R 6β , R 2α , R 3α , R 4α , R 5α and R 6α independently selected from hydrogen, halogen, nitro, alkyl, lower alkyl, alkenyl, alkynyl, carbocycle, cycloalkyl, cycloalkylalkyl, haloalkyl, aryl, aralkyl, heteroaryl group, heteroaryl-lower alkyl, heterocycle, heterocycle-lower alkyl, alkylthioalkyl, cycloalkylthioalkyl, arylthio-lower alkyl, aralkyl-lower-thioalkyl, hetero Arylthio-lower alkyl, heteroaralkyl-lower thioalkyl, heterocyclic thio-lower alkyl, heterocycloalkyl-lower thioalkyl, lower alkyl S(O)-lower alkyl, lower alkyl -S(O) 2 -lower alkyl, arylsulfinyl lower alkyl, arylsulfonyl lower alkyl, -C(O)R 2 , R 2 C(O)alkyl, aminoalkyl, cycloalkylaminoalkyl, arylamino lower alkyl, heteroarylamino lower alkyl, heterocyclic amino lower alkyl, hydroxyl, hydroxy...
Embodiment 1
[0860]
[0861] 1-(2,2-Dihydroxymethylbenzo[1,3]dioxol-5-yl)-3E-(3,4-Dimethoxy-5-thiophen-2-yl phenyl) propenone
[0862] Ex-1A: Catechol (2.2 g, 20 mmol) was dissolved in acetone. Diethyl dibromomalonate (7.0 g, 22 mmol) and potassium carbonate (2.76 g) were added, and the mixture was stirred at room temperature overnight. The solvent was separated under reduced pressure, and water was added to the residue. The residue was extracted with dichloromethane, the organic phase was washed with brine, dried over magnesium sulfate and evaporated. Chromatography (hexane / ethyl acetate, 4:1) gave 3.9 g of diethyl benzo[1,3]dioxole-2,2-dicarboxylate. 1 H-NMR (CDCl 3 ) δ6.90-6.97 (m, 4H), 4.37 (q, J = 7Hz, 4H), 1.32 (t, J = 7Hz, 6H).
[0863] Ex-1B: [bis(ethoxycarbonyl)methyldenedioxy]benzene (3.9 g, 14.7 mmol) obtained from Ex-1A was dissolved in THF (100 mL) and cooled with ice water. Lithium aluminum hydride (1M in THF, 44 mL) was added dropwise, and the mixture was stirred ov...
Embodiment 2
[0868]
[0869] 1-(2,2-Dihydroxymethylbenzo[1,3]dioxol-5-yl)-3E-(4-thiophen-2-ylphenyl)propenone
[0870] Ex-2A: 4-(thiophen-2-yl)benzaldehyde was obtained from 4-bromobenzaldehyde following a similar procedure as described in Ex-ID. 1 H-NMR (CDCl 3 )δ10.00(s, 1H), 7.88(d, J=9Hz, 2H), 7.77(d, J=9Hz, 2H), 7.46(d, J=4Hz, 1H), 7.39-7.41(m, 1H ), 7.12-7.15 (m, 1H).
[0871] Diethyl 5-acetylbenzo[1,3]dioxol-2,2-dicarboxylate from Ex-1C and 4-(thiophen-2-yl)benzaldehyde from Ex-2A Condensation was carried out in a similar manner as described in Ex-1 to obtain the title compound. Yellow solid, mp 166-168°C, 23.6% yield. 1 H-NMR (CDCl 3 )δ7.77(d, J=15Hz, 1H), 7.60-7.65(m, 5H), 7.51(d, J=2Hz, 1H), 7.45(d, J=15Hz, 1H), 7.37-7.38(m , 1H), 7.32 (d, J=5Hz, 1H), 7.09 (dd, J=4, 5Hz, 1H), 6.88 (d, J=8Hz, 1H), 3.96 (d, J=7Hz, 4H). MS m / z=394 ([M] + , 50%), 363 (100%). HRMS (EI) calculated value C 22 h 18 o 5 S: 394.0875. Measured value: 394.0869.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com