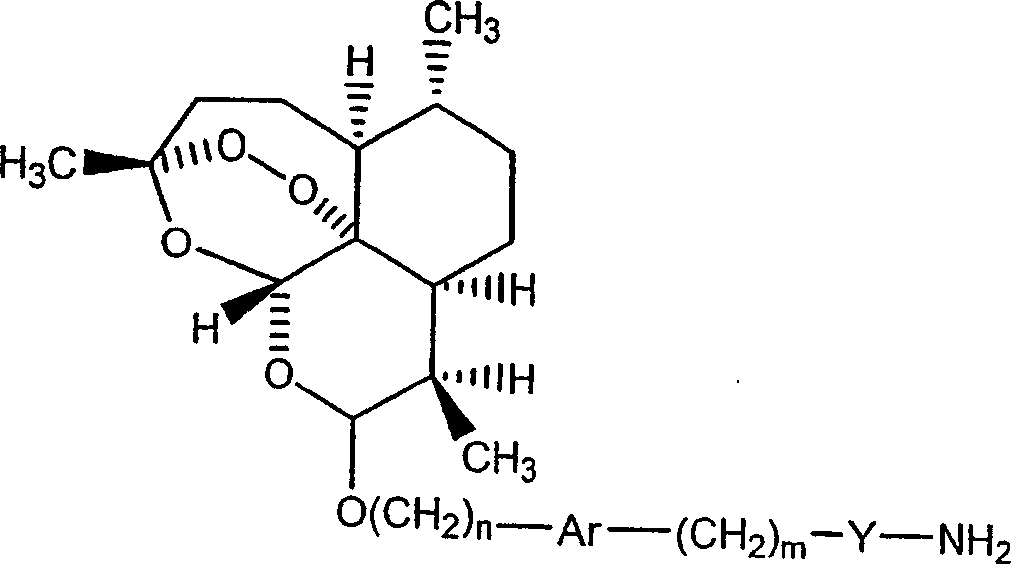
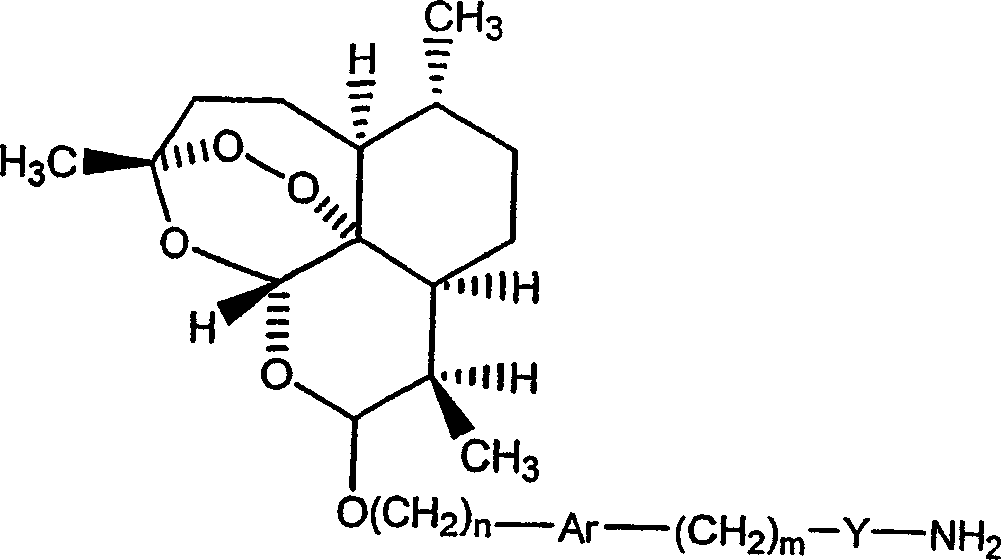
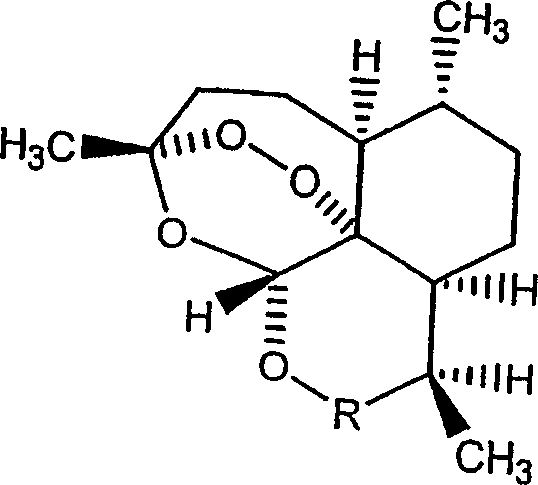
Covalent conjugates between artemisinin-related endoperoxides and iron-carrying proteins and methods of use
A peroxide, correlation technology, applied in the direction of animal/human protein, transferrin, peptide/protein composition, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] This example describes the preparation of representative compositions of the invention comprising one or more artemisinin-related endoperoxide molecules covalently linked to a holotransferrin molecule.
[0089] Synthesis of Artelinic acid hydrazide (ART-NH-NH 2 ): Attempts to prepare hydrazide derivatives of artesunate were unsuccessful, resulting in the formation of dihydroartemisinin due to a cyclization reaction. Atriptyline esters were synthesized from dihydroartemisinin as described above (Shrimali et al. (1998) Indian J Chem. 37B: 1161-1163). Artelinic acid (0.1 g, 0.24 mmol) was dissolved in anhydrous acetonitrile (0.48 mL). To this solution was added 1-hydroxybenzotriazole (HOBt) (0.038 g, 0.29 mmol), followed by ethyldimethylethylcarbodiimide (0.055 g, 0.29 mmol). The reaction mixture was stirred at room temperature and monitored by thin layer chromatography (TLC) until all the acid was converted to the HOBt ester.
[0090] A solution of hydrazine (0.46 mL, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com