Crystal for oral solid drug and oral solid drug for dysuria treatment containing the same
A technology for dysuria and solids, which can be used in drug combinations, pharmaceutical formulations, urinary system diseases, etc., and can solve the problems of unreported KMD-3213 crystalline polymorphic methods, unreported and mentioned which types of crystalline forms exist, preparation methods and its nature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
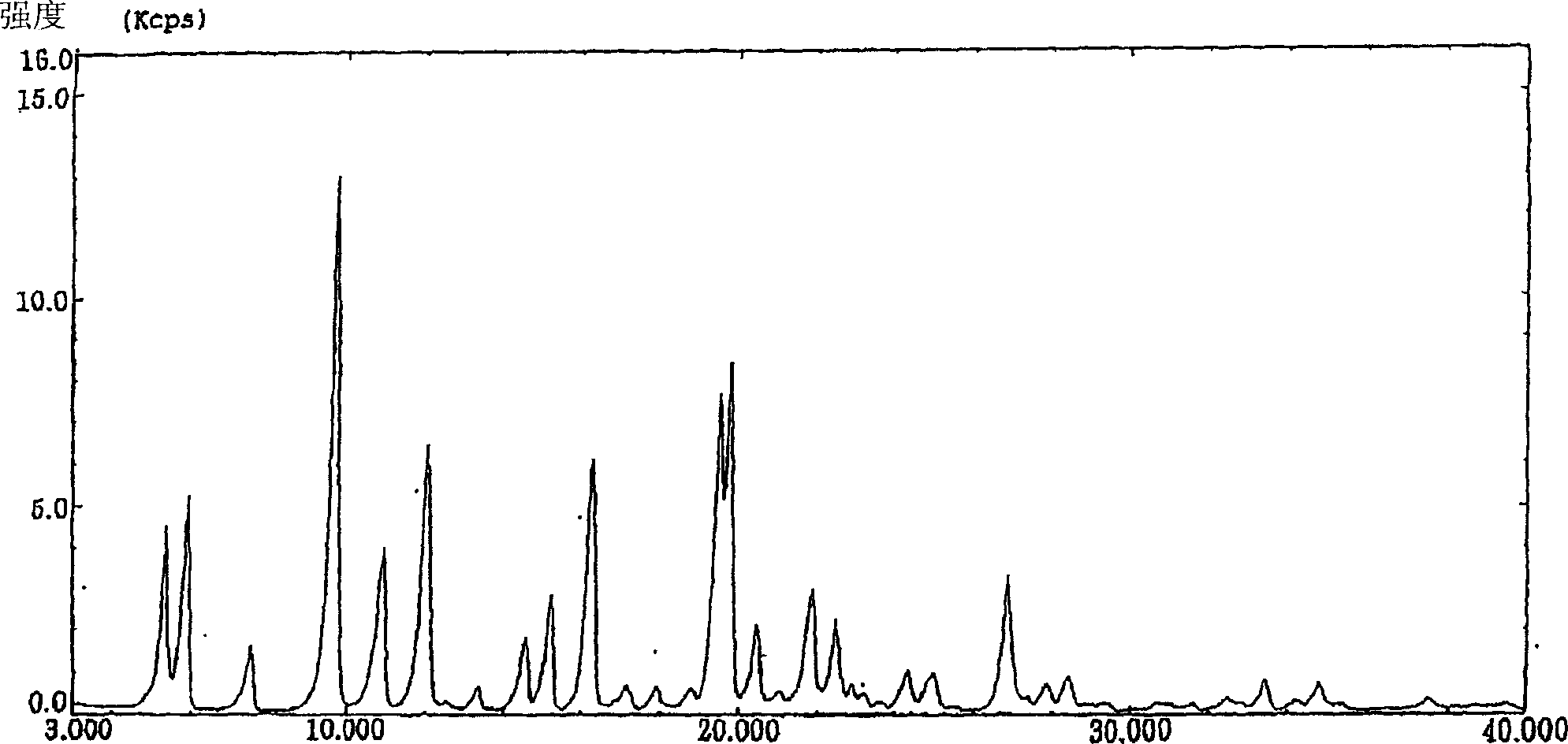
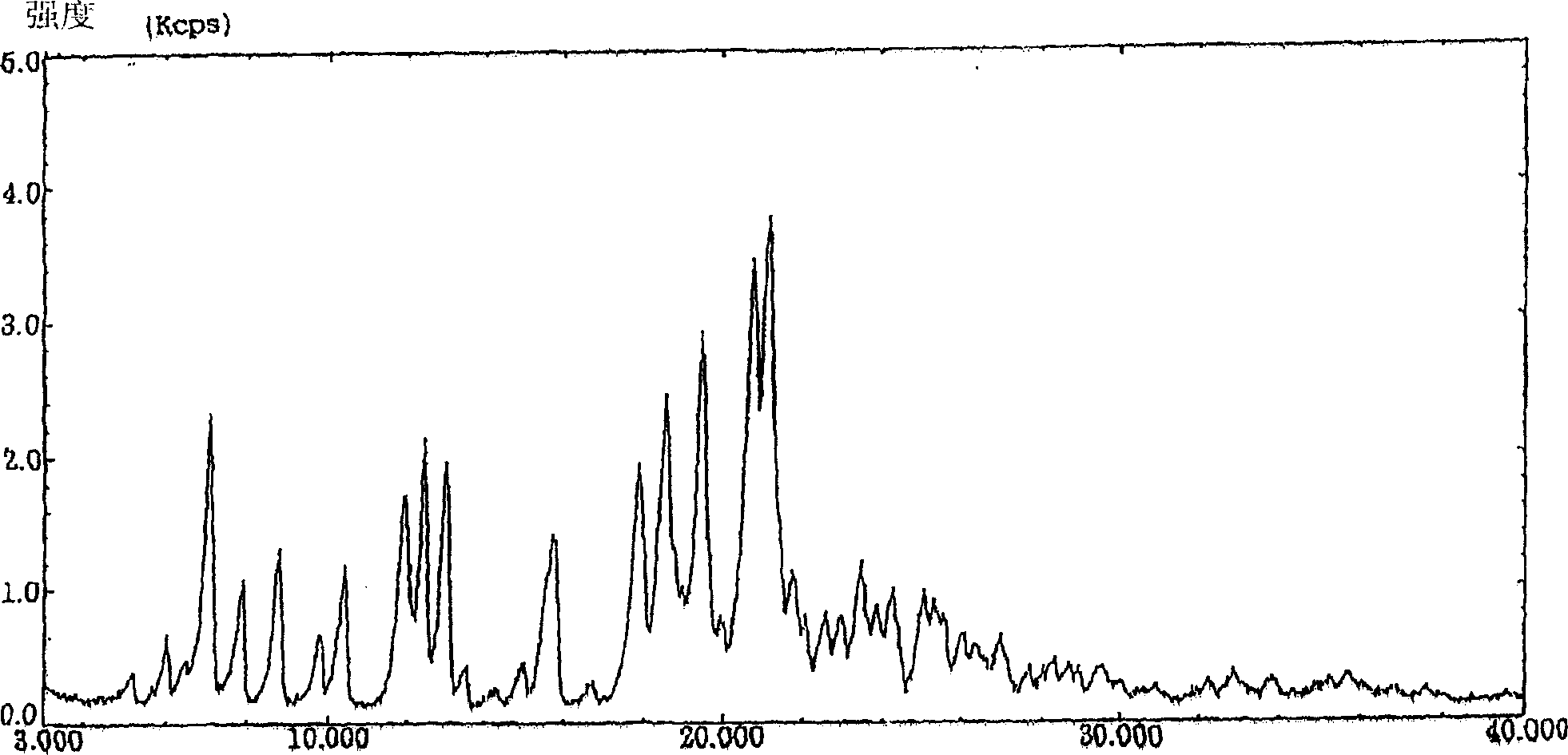
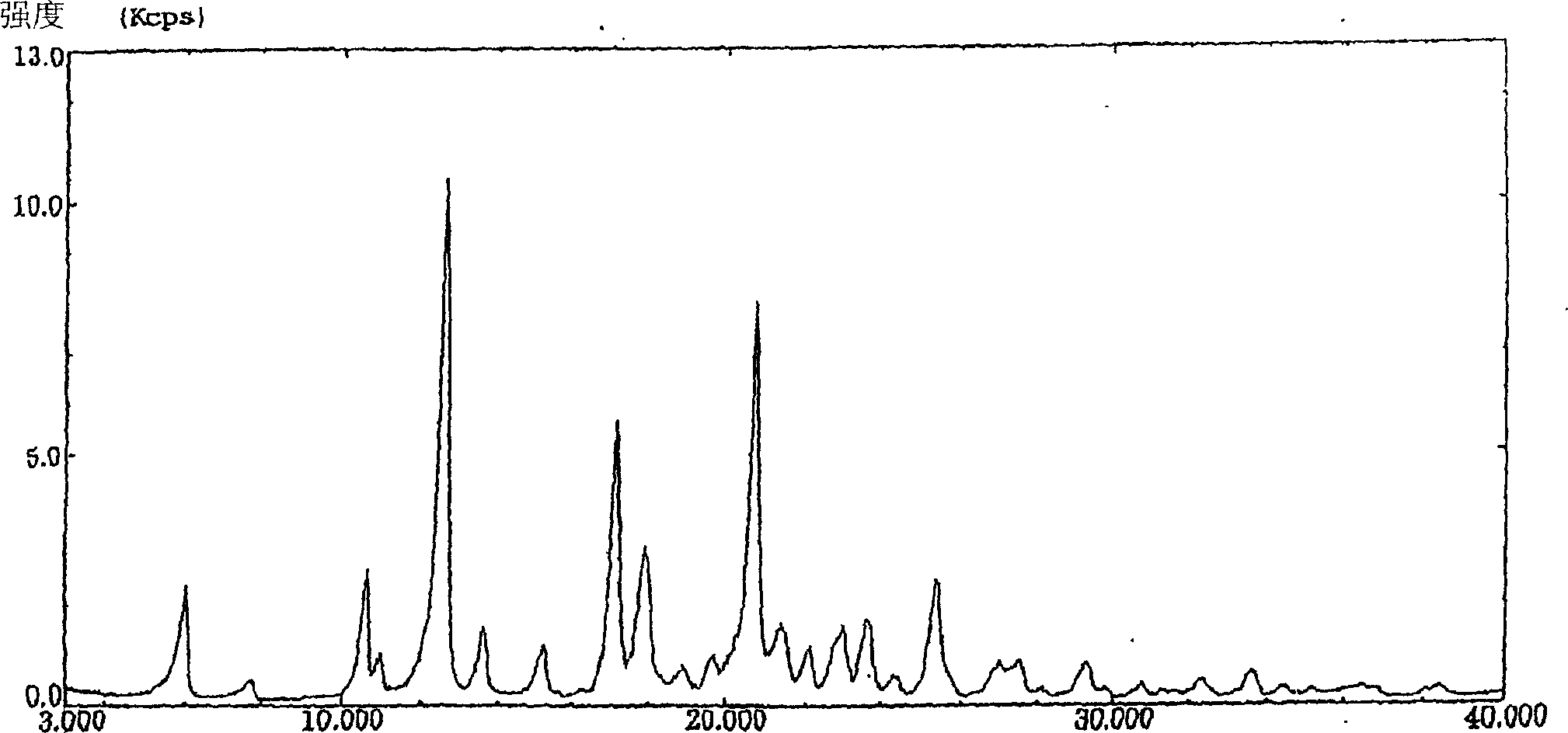
Image
Examples
Embodiment 1
[0051] Preparation of Form α
[0052] To 1 g of crude crystals of KMD-3213 was added 3 ml of ethyl acetate, and the mixture was heated to dissolve. After filtering off insoluble materials, the filtrate was left standing at room temperature. After the resulting crystals were completely precipitated, 10 ml of ethyl acetate was added thereto. The resulting crystals were collected by filtration and dried under vacuum at 50°C for 16 hours to yield 930 mg of Form α.
Embodiment 2
[0054] Preparation of Form β
[0055] To 1 g of crude crystals of KMD-3213 was added 0.4 ml of methanol, and the mixture was heated to dissolve. After filtering off the insoluble matter, 20 ml of petroleum ether was added thereto and vigorously shaken. The resulting crystals were collected by filtration and dried under vacuum at 50°C for 16 hours to yield 930 mg of Form α.
Embodiment 3
[0057] Preparation of Crystal Formsγ
[0058] To 1 g of crude crystals of KMD-3213 was added 4 ml of toluene, and the mixture was heated to dissolve. After filtering off insoluble materials, the filtrate was left standing at room temperature. After the resulting crystals were completely precipitated, 10 ml of toluene was added thereto. The resulting crystals were collected by filtration and dried under vacuum at 50°C for 16 hours to yield 970 mg of Form α.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com