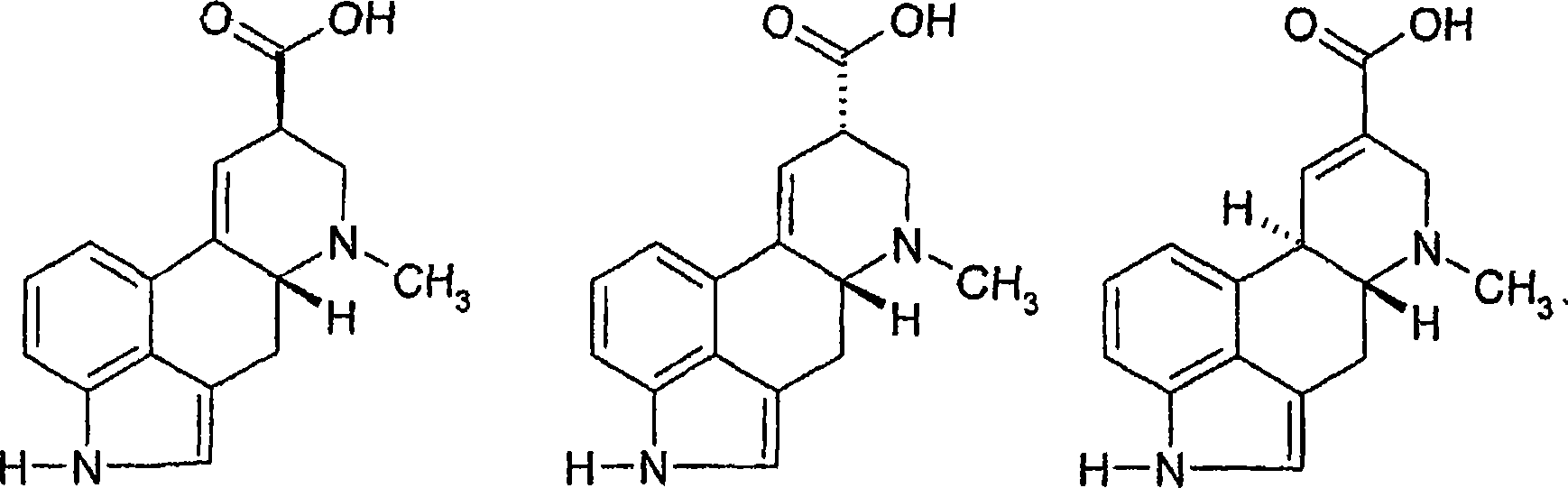
Process for the manufacture of lysergic acid
A manufacturing method and technology of lysergic acid, applied in the direction of organic chemistry, etc., can solve the problem of low concentration of paspalicacid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of lysergic acid without recycling mother liquor
[0029] Paspalic acid (100.0 g) (98.5% by titration) was dissolved in 5% aqueous sodium hydroxide solution (1000 mL), and sodium hydroxide (150 g) was added to the solution. A two-phase mixture was observed to form. The resulting biphasic mixture was then mixed for about 4 hours at about 50°C under nitrogen. The reaction mixture was diluted with water (1000 mL), cooled to 10° C., and acidified to pH about 3.5 with 40% sulfuric acid. A suspension of crystalline lysergic acid sulfate formed and was mixed at about 5°C for about 2 hours. The crystalline lysergic acid sulfate was filtered off, extracted with a 95:5 (v / v) mixture of methanol and ammonia (3 x 500 mL), the combined extract was evaporated to about 200 g, diluted with water (200 mL), and crystallized at about 5 °C 24 hours. Crystalline lysergic acid was then isolated and washed with water (100 mL) and methanol (3 x 100 mL). After drying...
Embodiment 2
[0031] Example 2: Preparation of Lysergic Acid with Mother Liquor Circulation
[0032] Sodium hydroxide (50 g) was dissolved in water (800 mL) and 200 mL of the concentrated mother liquor from Example 1. To this solution was added paspalic acid (100.0 g) (98.5% by titration) and finally sodium hydroxide (150 g). A biphasic reaction mixture was then formed and mixed for about 4 hours at about 50°C under nitrogen. The reaction mixture was diluted with water (1000 mL), cooled to 10 °C, and acidified to about pH 3.5 with 40% sulfuric acid. The resulting suspension was mixed for 2 hours at about 5°C and the crystalline lysergic acid sulfate was filtered off. Lysergic acid sulfate was extracted with a 95:5 (v / v) mixture of methanol and ammonia (3×500 mL). The combined extracts were evaporated to about 200 g, diluted with water (200 mL) and crystallized at about 5°C for 24 hours. Crystalline lysergic acid was then isolated and washed with water (100 mL) and methanol (3 x 100 mL)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com