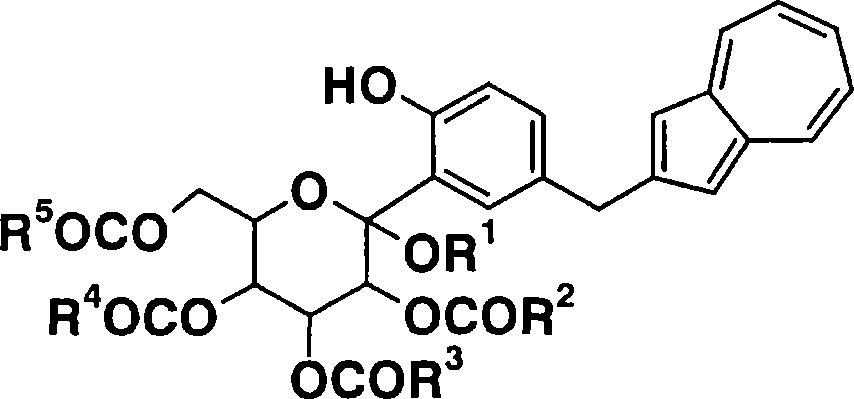
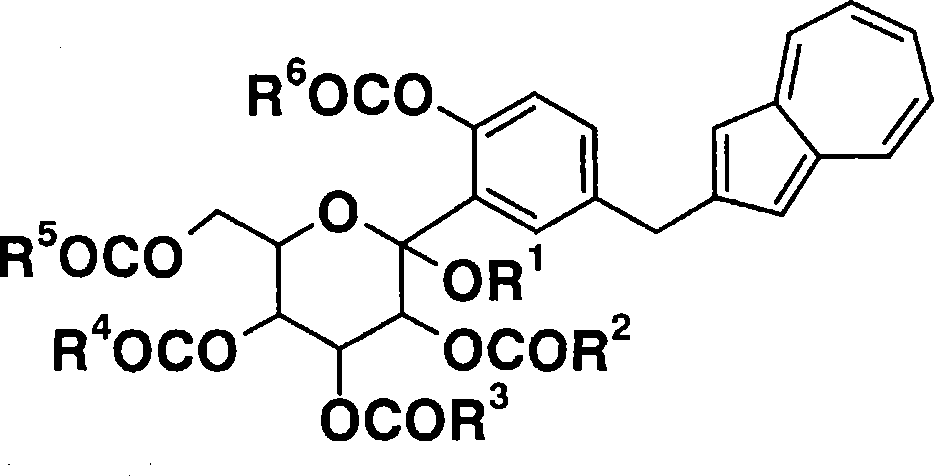
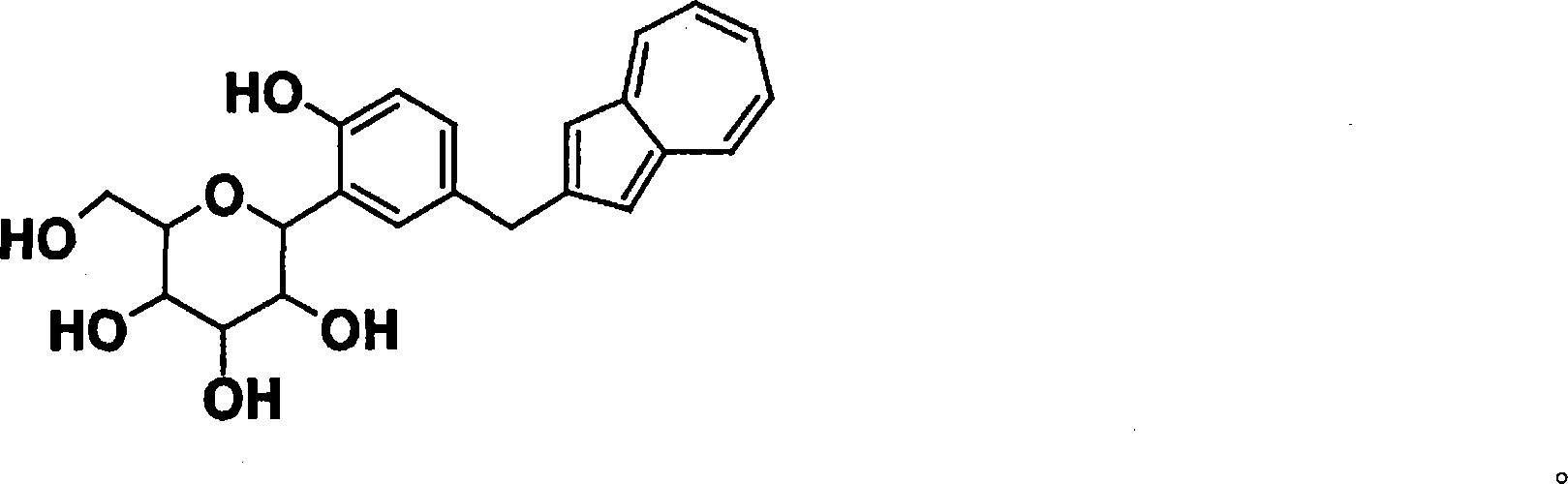
Process for production of azulene derivatives and intermediates for the synthesis of the same
A technology of derivatives and compounds, applied in the field of production of azulene derivatives and intermediates for the synthesis of the azulene derivatives, can solve the problems of production, cost and environmental protection that are not completely satisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0184] Synthesis of 1-[3-bromo-4-(methoxymethoxy)phenyl]acetone
[0185] To a solution of 1-(3-bromo-4-hydroxyphenyl)acetone (515.05 g, 2.25 mol) in acetone (2.58 L) was added potassium carbonate (466.61 g) and chloromethylmethyl ether (272.23 g). The mixture was stirred at -5°C to 5°C for 30 minutes. The reaction mixture was extracted by adding toluene (4.2 L) and water (4.2 L). The organic layer was washed with 0.1M aqueous sodium hydroxide solution (4.2 L), then washed twice with water (4.2 L). The solvent was distilled off under reduced pressure to obtain light yellow oily substance 1-[3-bromo-4-(methoxymethoxy)phenyl]acetone [605.25 g, yield=99%, purity=96% (HPLC )].
Embodiment 2
[0187] Synthesis of 1-[3-bromo-4-(methoxymethoxy)phenyl]acetone
[0188] To a solution of phosphorus pentoxide (1.70 g) in toluene (9 mL) was added methylal (3.04 g) and 1-(3-bromo-4-hydroxyphenyl)acetone (0.915 g, 3.99 mmol). The mixture was stirred at -5°C to 5°C for 17 hours. Water (9 mL) was added to extract the reaction mixture. The organic layer was washed twice with 10% potassium carbonate aqueous solution (9 mL), and then the solvent was distilled off under reduced pressure to obtain 1-[3-bromo-4-(methoxymethoxy)phenyl as a pale yellow oily substance ] Acetone [1.09 g, Yield = 100%, Purity = 94% (HPLC)].
Embodiment 3
[0190] Synthesis of 2-[3-bromo-4-(methoxymethoxy)benzyl]azulene
[0191] To a solution of 1-[3-bromo-4-(methoxymethoxy)phenyl]acetone (68.7 g, 251.54 mmol) in toluene (690 mL) was added pyrrolidine (41 mL). The mixture was subjected to atmospheric distillation, and 73 ml of the solvent was distilled off. Pyrrolidine (20 ml) was added, the mixture was subjected to atmospheric distillation, and 27 ml of the solvent was distilled off. The reaction mixture was cooled, and the solvent was distilled off under reduced pressure. To the obtained residue were added 2H-aryhepta[b]furan-2-one (40 g) and isopropanol (370 ml), and the mixture was refluxed with heating for 19 hours. The reaction mixture was concentrated. Toluene (690 ml) and 1M hydrochloric acid (690 ml) were added to the residue. After decanting the supernatant to remove the resulting insoluble precipitate, the aqueous layer and the organic layer were separated. Further 1M hydrochloric acid (690 mL) was added to the or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com