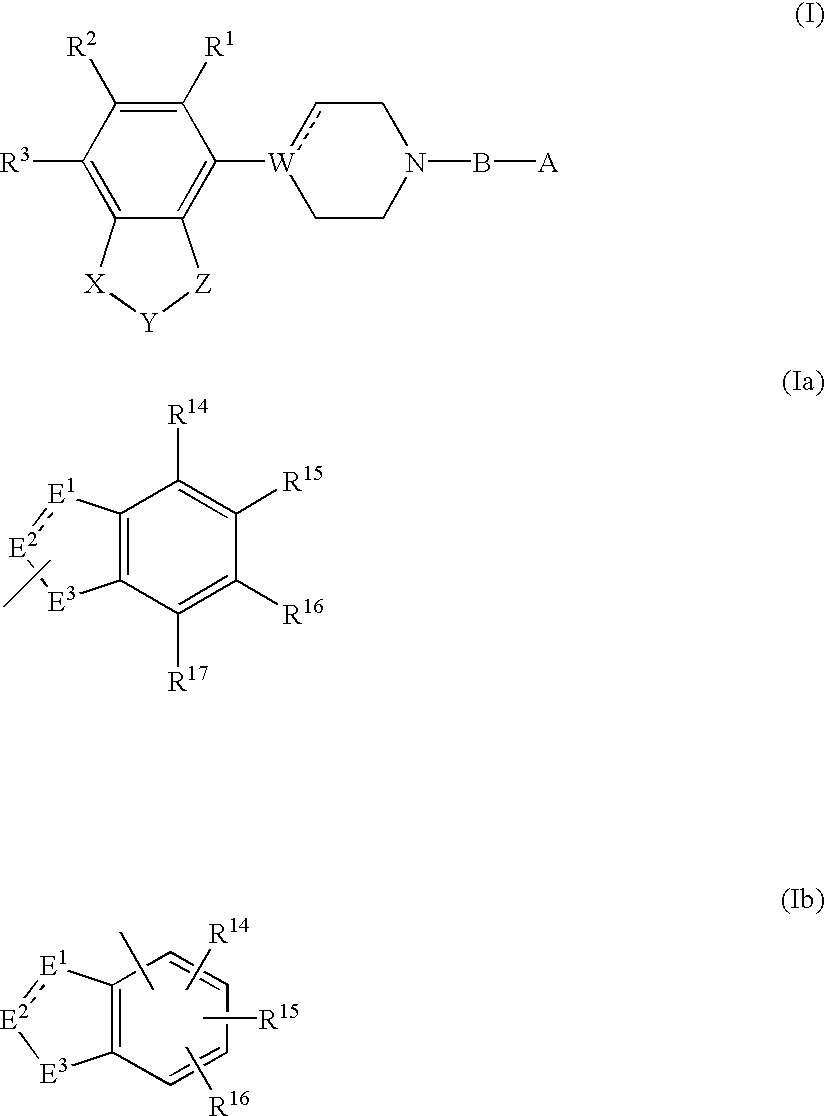
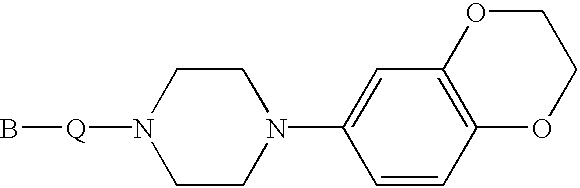
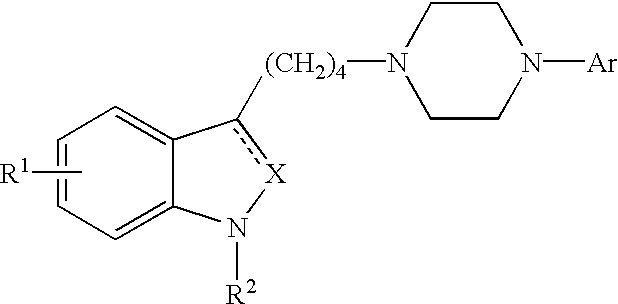
Piperidine, tetrahydropyridine and piperazine derivatives, their preparation and use
a technology of tetrahydropyridine and piperazine, which is applied in the field of piperidine, tetrahydropyridine and piperazine derivatives, their preparation and use, and can solve the problems of patients withdrawing from treatment, ssris and all other antidepressants currently available, and several weeks of treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0223] 1a, 1-[1,4-Benzodioxan-]5-yl-4-[-(inden-1-yl)-4-butyl]piperazine, oxalate
[0224] A mixture of 4(1-indenyl)butyl methanesulfonate (1.2 g, prepared as described in U.S. Pat. No. 5665725), 1-(1,4-benzodioxan-5-yl)piperazine (1.2 g), and potassium carbonate (0.8 g) in 3-methyl-2-pentanone (50 mL) was refluxed for 16 h. Cooling, filtration, and removal of solvent in vacuo gave an oil which was applied to silica gel flash chromatography (eluent: heptane / methylene chloride / triethylamine 70:26:4). The obtained oil was converted to the title oxalate salt (0.7 g) from acetone by addition of oxalic acid. Mp 130-31.degree. C. .sup.1H NMR (DMSO-d.sub.6): 1.60-1.90 (m, 4H); 2.55 (t, 2H); 3.05 (t, 2H); 3.15 (s, 8H); 3.35 (s, 8H); 3.35 (s, 2H); 4.15 4.35 (m, 4H); 6.30 (s, 1H); 6.50 (t, 1H); 6.60 (s, 1H); 6.75 (t, 1H); 7.20 (t, 1H); 7.25(t, 1H); 1H); 7.40 (d, 1H); 7.50 (d, 1H). MS m / z (%):391 (MH+, 79%), 218 (37%), 162 (50%), 129 (100%)
[0225] The following compounds were prepared analogously (...
example 2
[0230] 2a, 1-[1,4-Benzodioxan-5-yl]-4-[2-(5-fluorobenzofuran-3-yl)ethyl]pi-perazine, oxalate
[0231] A solution of 5-fluorobenzofuran-3-carboxylic acid (56 g) and saturated etheral solution of hydrochloric gas (300 mL) in methanol (600 mL) was stirred for 16 h at room temperature. Further etheral HCl was added (300 mL) followed by stirring for 24 h. Concentration in vacuo gave a dark crystalline material, methyl 5-fluorobenzofuran-3-carboxylate (58 g). Lithium aluminium hydride (15 g) was suspended in tetrahydrofuran (400 mL) under a nitrogen atmosphere followed by dropwise addition of a solution of methyl 5-fluorobenzofuran-3-carboxylate (58 g) in tetrahydrofuran (300 mL). The temperature increased to 55.degree. C. during the addition. After stirring for 2 h the reaction was quenched successively with water (30 mL), 15% aq. sodium hydroxide (15 mL), and water (75 mL). Further tetrahydrofuran (500 mL) was added and the mixture stirred for 1 h. The mixture was filtered and the precipit...
example 3
[0241] 3a, 1-[Benzofuran-7-yl]-4-[2(5-fluorobenzofuran-3-yl)ethyl]piprazin-e, fumarate
[0242] A solution of 5-fluorobenzofuran-3-ylacetic acid (4.6 g, prepared as described in Example 2) in dry tetrahydrofuran (200 mL) was added dropwise to a suspension of lithium aluminium hydride (4.5 g) in dry tetrahydrofuran (150 mL) at room temperature. After reflux for 2 h the reaction was quenched by successive additions of water (9.2 mL), 15% aq. sodium hydroxide (4.6 mL), water (23 mL). Filtration and removal of solvent in vacuo gave an oil, 2-(5-fluorobenzofuran-3-yl)ethanol (4.3 g).
[0243] A solution of 2-(5-fluorobenzofuran-3-yl)ethanol (5.2 g) and tetrabromomethane (11.6 g) in acetonitrile (175 mL) was treated with tnrphenylphosphine (8.3 g) at 0.degree. C. After stirring for 15 min, the mixture was concentrated in vacuo an the resulting oil was applied to a silica gel flash column (eluent: heptaneiethyl acetate 65:35) resulting in an oil, 3(2-bromoethyl)-5-fluorobenzofuran (7.4 g).
[0244]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com